From vaccines to memory and back
- PMID: 21029957
- PMCID: PMC3760154
- DOI: 10.1016/j.immuni.2010.10.008
From vaccines to memory and back
Abstract
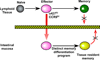
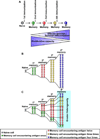
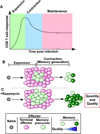
Vaccines work by eliciting an immune response and consequent immunological memory that mediates protection from infection or disease. Recently, new methods have been developed to dissect the immune response in experimental animals and humans, which have led to increased understanding of the molecular mechanisms that control differentiation and maintenance of memory T and B cells. In this review we will provide an overview of the cellular organization of immune memory and underline some of the outstanding questions on immunological memory and how they pertain to vaccination strategies. Finally we will discuss how we can learn about antigen design from the interrogation of our memory T and B cells-a journey from vaccines to memory and back.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures


References
-
- Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. - PubMed
-
- Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. - PubMed
-
- Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

