Vaccination strategies to promote mucosal antibody responses
- PMID: 21029959
- PMCID: PMC3045856
- DOI: 10.1016/j.immuni.2010.09.013
Vaccination strategies to promote mucosal antibody responses
Abstract
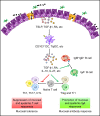
There are great interest and demand for the development of vaccines to prevent and treat diverse microbial infections. Mucosal vaccines elicit immune protection by stimulating the production of antibodies at mucosal surfaces and systemic districts. Being positioned in close proximity to a large community of commensal microbes, the mucosal immune system deploys a heterogeneous population of cells and a complex regulatory network to maintain the balance between surveillance and tolerance. A successful mucosal vaccine relies on leveraging the functions of these immune cells and regulatory components. We review the important cellular interactions and molecular pathways underlying the induction and regulation of mucosal antibody responses and discuss their implications on mucosal vaccination.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures

References
-
- Abreu M.T. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat. Rev. Immunol. 2010;10:131–144. - PubMed
-
- Appelberg R. Neutrophils and intracellular pathogens: beyond phagocytosis and killing. Trends Microbiol. 2007;15:87–92. - PubMed
-
- Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat. Rev. Immunol. 2008;8:411–420. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

