Towards improved animal models of neonatal white matter injury associated with cerebral palsy
- PMID: 21030421
- PMCID: PMC2965396
- DOI: 10.1242/dmm.002915
Towards improved animal models of neonatal white matter injury associated with cerebral palsy
Abstract
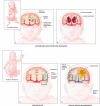
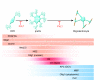
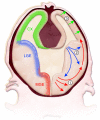
Newborn neurological injuries are the leading cause of intellectual and motor disabilities that are associated with cerebral palsy. Cerebral white matter injury is a common feature in hypoxic-ischemic encephalopathy (HIE), which affects full-term infants, and in periventricular leukomalacia (PVL), which affects preterm infants. This article discusses recent efforts to model neonatal white matter injury using mammalian systems. We emphasize that a comprehensive understanding of oligodendrocyte development and physiology is crucial for obtaining new insights into the pathobiology of HIE and PVL as well as for the generation of more sophisticated and faithful animal models.
Figures
Similar articles
-
The vulnerable oligodendrocyte: inflammatory observations on a cause of cerebral palsy.Neurology. 2001 May 22;56(10):1254-5. doi: 10.1212/wnl.56.10.1254. Neurology. 2001. PMID: 11376167 Review. No abstract available.
-
Diffusion tensor imaging in children with periventricular leukomalacia: variability of injuries to white matter tracts.AJNR Am J Neuroradiol. 2007 Aug;28(7):1213-22. doi: 10.3174/ajnr.A0534. AJNR Am J Neuroradiol. 2007. PMID: 17698519 Free PMC article.
-
Nitrosative and oxidative injury to premyelinating oligodendrocytes in periventricular leukomalacia.J Neuropathol Exp Neurol. 2003 May;62(5):441-50. doi: 10.1093/jnen/62.5.441. J Neuropathol Exp Neurol. 2003. PMID: 12769184
-
Cross-sectional comparison of periventricular leukomalacia in preterm and term children.Neurology. 2010 Apr 27;74(17):1386-91. doi: 10.1212/WNL.0b013e3181dad62d. Neurology. 2010. PMID: 20421583
-
Periventricular leukomalacia: overview and recent findings.Pediatr Dev Pathol. 2006 Jan-Feb;9(1):3-13. doi: 10.2350/06-01-0024.1. Epub 2006 Apr 4. Pediatr Dev Pathol. 2006. PMID: 16808630 Review.
Cited by
-
Impact of early brain lesions on the optic radiations in children with cerebral palsy.Front Neurosci. 2022 Oct 5;16:924938. doi: 10.3389/fnins.2022.924938. eCollection 2022. Front Neurosci. 2022. PMID: 36278011 Free PMC article.
-
Behavioral and histological outcomes following neonatal HI injury in a preterm (P3) and term (P7) rodent model.Behav Brain Res. 2014 Feb 1;259:85-96. doi: 10.1016/j.bbr.2013.10.038. Epub 2013 Nov 1. Behav Brain Res. 2014. PMID: 24185032 Free PMC article.
-
White matter injury in the preterm infant: pathology and mechanisms.Acta Neuropathol. 2017 Sep;134(3):331-349. doi: 10.1007/s00401-017-1718-6. Epub 2017 May 22. Acta Neuropathol. 2017. PMID: 28534077 Free PMC article. Review.
-
Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination.Nat Neurosci. 2011 Jun 26;14(8):1009-16. doi: 10.1038/nn.2855. Nat Neurosci. 2011. PMID: 21706018 Free PMC article.
-
Early-stage effect of HIBD on neuro-motor function and organic composition of neurovascular units in neonatal rats.Front Neurosci. 2023 Nov 21;17:1242936. doi: 10.3389/fnins.2023.1242936. eCollection 2023. Front Neurosci. 2023. PMID: 38075277 Free PMC article.
References
-
- Alix JJ, Fern R. (2009). Glutamate receptor-mediated ischemic injury of premyelinated central axons. Ann Neurol. 66, 682–693 - PubMed
-
- Althaus HH, Kloppner S, Klopfleisch S, Schmitz M. (2008). Oligodendroglial cells and neurotrophins: a polyphonic cantata in major and minor. J Mol Neurosci. 35, 65–79 - PubMed
-
- Anjari M, Counsell SJ, Srinivasan L, Allsop JM, Hajnal JV, Rutherford MA, Edwards AD. (2009). The association of lung disease with cerebral white matter abnormalities in preterm infants. Pediatrics 124, 268–276 - PubMed
-
- Anthony TE, Heintz N. (2007). The folate metabolic enzyme ALDH1L1 is restricted to the midline of the early CNS, suggesting a role in human neural tube defects. J Comp Neurol. 500, 368–383 - PubMed
-
- Arnett HA, Fancy SP, Alberta JA, Zhao C, Plant SR, Kaing S, Raine CS, Rowitch DH, Franklin RJ, Stiles CD. (2004). bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science 306, 2111–2115 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

