Mapping of long-range associations throughout the fission yeast genome reveals global genome organization linked to transcriptional regulation
- PMID: 21030438
- PMCID: PMC3001101
- DOI: 10.1093/nar/gkq955
Mapping of long-range associations throughout the fission yeast genome reveals global genome organization linked to transcriptional regulation
Abstract
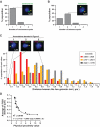
We have comprehensively mapped long-range associations between chromosomal regions throughout the fission yeast genome using the latest genomics approach that combines next generation sequencing and chromosome conformation capture (3C). Our relatively simple approach, referred to as enrichment of ligation products (ELP), involves digestion of the 3C sample with a 4 bp cutter and self-ligation, achieving a resolution of 20 kb. It recaptures previously characterized genome organizations and also identifies new and important interactions. We have modeled the 3D structure of the entire fission yeast genome and have explored the functional relationships between the global genome organization and transcriptional regulation. We find significant associations among highly transcribed genes. Moreover, we demonstrate that genes co-regulated during the cell cycle tend to associate with one another when activated. Remarkably, functionally defined genes derived from particular gene ontology groups tend to associate in a statistically significant manner. Those significantly associating genes frequently contain the same DNA motifs at their promoter regions, suggesting that potential transcription factors binding to these motifs are involved in defining the associations among those genes. Our study suggests the presence of a global genome organization in fission yeast that is functionally similar to the recently proposed mammalian transcription factory.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

