Using propensity score modeling to minimize the influence of confounding risks related to prenatal tobacco exposure
- PMID: 21030468
- PMCID: PMC2991623
- DOI: 10.1093/ntr/ntq170
Using propensity score modeling to minimize the influence of confounding risks related to prenatal tobacco exposure
Abstract
Introduction: Despite efforts to control for confounding variables using stringent sampling plans, selection bias typically exists in observational studies, resulting in unbalanced comparison groups. Ignoring selection bias can result in unreliable or misleading estimates of the causal effect.
Methods: Generalized boosted models were used to estimate propensity scores from 42 confounding variables for a sample of 361 neonates. Using emergent neonatal attention and orientation skills as an example developmental outcome, we examined the impact of tobacco exposure with and without accounting for selection bias. Weight at birth, an outcome related to tobacco exposure, also was used to examine the functionality of the propensity score approach.
Results: Without inclusion of propensity scores, tobacco-exposed neonates did not differ from their nonexposed peers in attention skills over the first month or in weight at birth. When the propensity score was included as a covariate, exposed infants had marginally lower attention and a slower linear change rate at 4 weeks, with greater quadratic deceleration over the first month. Similarly, exposure-related differences in birth weight emerged when propensity scores were included as a covariate.
Conclusions: The propensity score method captured the selection bias intrinsic to this observational study of prenatal tobacco exposure. Selection bias obscured the deleterious impact of tobacco exposure on the development of neonatal attention. The illustrated analytic strategy offers an example to better characterize the impact of prenatal tobacco exposure on important developmental outcomes by directly modeling and statistically accounting for the selection bias from the sampling process.
Figures
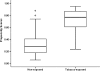

References
-
- Anda R, Williamson D, Escobedo L, Mast E, Giovino G, Remington P. Depression and the dynamics of smoking: A national perspective. Journal of the American Medical Association. 1990;264:1541–1545. - PubMed
-
- Baghurst P, Tong S, Woodward A, McMichael A. Effects of maternal smoking upon neuropsychological development in early childhood: Importance of taking account of social and environmental factors. Paediatric and Perinatal Epidemiology. 1992;6:403–415. - PubMed
-
- Braitman L, Rosenbaum P. Rare outcomes, common treatments: Analytic strategies using propensity scores. Annals of Internal Medicine. 2002;137:693–695. - PubMed
-
- Breiman L, Friedman JH, Olshen RA, Stone CJ. Classification and regression trees. Belmont, CA: Wadsworth International Group; 1984.
-
- Breslau N. Psychiatric comorbidity of smoking and nicotine dependence. Behavior Genetics. 1995;25:95–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

