Ewing sarcoma gene Ews regulates hematopoietic stem cell senescence
- PMID: 21030557
- PMCID: PMC3056469
- DOI: 10.1182/blood-2010-04-279349
Ewing sarcoma gene Ews regulates hematopoietic stem cell senescence
Abstract
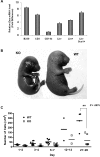
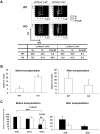
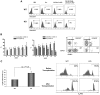
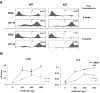
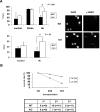
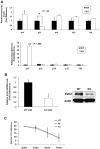
The longevity of organisms is maintained by stem cells. If an organism loses the ability to maintain a balance between quiescence and differentiation in the stem/progenitor cell compartment due to aging and/or stress, this may result in death or age-associated diseases, including cancer. Ewing sarcoma is the most lethal bone tumor in young patients and arises from primitive stem cells. Here, we demonstrated that endogenous Ewing sarcoma gene (Ews) is indispensable for stem cell quiescence, and that the ablation of Ews promotes the early onset of senescence in hematopoietic stem progenitor cells. The phenotypic and functional changes in Ews-deficient stem cells were accompanied by an increase in senescence-associated β-galactosidase staining and a marked induction of p16(INK4a) compared with wild-type counterparts. With its relevance to cancer and possibly aging, EWS is likely to play a significant role in maintaining the functional capacity of stem cells and may provide further insight into the complexity of Ewing sarcoma in the context of stem cells.
Figures

References
-
- Meltzer PS. Is Ewing's sarcoma a stem cell tumor? Cell Stem Cell. 2007;1(1):13–15. - PubMed
-
- Bertolotti A, Bell B, Tora L. The N-terminal domain of human TAFII68 displays transactivation and oncogenic properties. Oncogene. 1999;18(56):8000–8010. - PubMed
-
- Matsunobu T, Tanaka K, Nakamura T, et al. The possible role of EWS-Fli1 in evasion of senescence in Ewing's family tumors. Cancer Res. 2006;66(2):803–811. - PubMed
-
- Deneen B, Denny CT. Loss of p16 pathways stabilizes EWS/FLI1 expression and complements EWS/FLI1 mediated transformation. Oncogene. 2001;20(46):6731–6741. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

