Blockade of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury
- PMID: 21030600
- PMCID: PMC3014038
- DOI: 10.1681/ASN.2009121236
Blockade of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury
Abstract
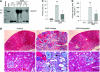
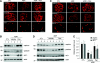
Recent studies implicate Wnt/β-catenin signaling in podocyte dysfunction. Because vitamin D analogs can inhibit β-catenin in other tissues, we tested whether the vitamin D analog paricalcitol could ameliorate podocyte injury, proteinuria, and renal fibrosis in adriamycin (ADR) nephropathy. Compared with vehicle-treated controls, paricalcitol preserved expression of nephrin, podocin, and WT1; prevented proteinuria; and reduced glomerulosclerotic lesions induced by ADR. Paricalcitol also inhibited expression of proinflammatory cytokines, reduced renal infiltration of monocytes/macrophages, hampered activation of renal myofibroblasts, and suppressed expression of the fibrogenic TGF-β1, CTGF, fibronectin, and types I and III collagen. Selective suppression of renal Wnt4, Wnt7a, Wnt7b, and Wnt10a expression after ADR accompanied these renoprotective effects of paricalcitol. Significant upregulation of β-catenin, predominantly in podocytes and tubular epithelial cells, accompanied renal injury; paricalcitol largely abolished this induction of renal β-catenin and inhibited renal expression of Snail, a downstream effector of Wnt/β-catenin signaling. Administration of paricalcitol also ameliorated established proteinuria. In vitro, paricalcitol induced a physical interaction between the vitamin D receptor and β-catenin in podocytes, which led to suppression of β-catenin-mediated gene transcription. In summary, these findings suggest that paricalcitol prevents podocyte dysfunction, proteinuria, and kidney injury in adriamycin nephropathy by inhibiting Wnt/β-catenin signaling.
Figures




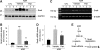


Similar articles
-
Canonical Wnt/β-catenin signaling mediates transforming growth factor-β1-driven podocyte injury and proteinuria.Kidney Int. 2011 Dec;80(11):1159-1169. doi: 10.1038/ki.2011.255. Epub 2011 Aug 10. Kidney Int. 2011. PMID: 21832980 Free PMC article.
-
Salidroside ameliorates Adriamycin nephropathy in mice by inhibiting β-catenin activity.J Cell Mol Med. 2019 Jun;23(6):4443-4453. doi: 10.1111/jcmm.14340. Epub 2019 Apr 16. J Cell Mol Med. 2019. PMID: 30993911 Free PMC article.
-
VDR Activation Reduces Proteinuria and High-Glucose-Induced Injury of Kidneys and Podocytes by Regulating Wnt Signaling Pathway.Cell Physiol Biochem. 2017;43(1):39-51. doi: 10.1159/000480315. Epub 2017 Aug 24. Cell Physiol Biochem. 2017. PMID: 28848172
-
Wnt/β-catenin signalling and podocyte dysfunction in proteinuric kidney disease.Nat Rev Nephrol. 2015 Sep;11(9):535-45. doi: 10.1038/nrneph.2015.88. Epub 2015 Jun 9. Nat Rev Nephrol. 2015. PMID: 26055352 Free PMC article. Review.
-
Vitamin D and proteinuria: a critical review of molecular bases and clinical experience.Nefrologia. 2013;33(5):716-26. doi: 10.3265/Nefrologia.pre2013.Apr.12025. Nefrologia. 2013. PMID: 24089164 Review. English, Spanish.
Cited by
-
Four-and-a-Half LIM Domains Protein 2 Is a Coactivator of Wnt Signaling in Diabetic Kidney Disease.J Am Soc Nephrol. 2015 Dec;26(12):3072-84. doi: 10.1681/ASN.2014100989. Epub 2015 Apr 8. J Am Soc Nephrol. 2015. PMID: 25855776 Free PMC article.
-
Key Fibrogenic Signaling.Curr Pathobiol Rep. 2015;3(2):183-192. doi: 10.1007/s40139-015-0077-z. Curr Pathobiol Rep. 2015. PMID: 25973345 Free PMC article. Review.
-
Wnt/β-catenin signaling is hyperactivated in systemic sclerosis and induces Smad-dependent fibrotic responses in mesenchymal cells.Arthritis Rheum. 2012 Aug;64(8):2734-45. doi: 10.1002/art.34424. Arthritis Rheum. 2012. PMID: 22328118 Free PMC article.
-
Klotho attenuates diabetic nephropathy in db/db mice and ameliorates high glucose-induced injury of human renal glomerular endothelial cells.Cell Cycle. 2019 Mar-Apr;18(6-7):696-707. doi: 10.1080/15384101.2019.1580495. Epub 2019 Mar 17. Cell Cycle. 2019. PMID: 30784349 Free PMC article.
-
Complement component C5a induces aberrant epigenetic modifications in renal tubular epithelial cells accelerating senescence by Wnt4/βcatenin signaling after ischemia/reperfusion injury.Aging (Albany NY). 2019 Jul 8;11(13):4382-4406. doi: 10.18632/aging.102059. Aging (Albany NY). 2019. PMID: 31284268 Free PMC article.
References
-
- Shankland SJ: The podocyte's response to injury: Role in proteinuria and glomerulosclerosis. Kidney Int 69: 2131–2147, 2006 - PubMed
-
- Wiggins RC: The spectrum of podocytopathies: A unifying view of glomerular diseases. Kidney Int 71: 1205–1214, 2007 - PubMed
-
- Patrakka J, Tryggvason K: New insights into the role of podocytes in proteinuria. Nat Rev Nephrol 5: 463–468, 2009 - PubMed
-
- Abbate M, Zoja C, Remuzzi G: How does proteinuria cause progressive renal damage? J Am Soc Nephrol 17: 2974–2984, 2006 - PubMed
-
- Zandi-Nejad K, Eddy AA, Glassock RJ, Brenner BM: Why is proteinuria an ominous biomarker of progressive kidney disease? Kidney Int Suppl S76–S89, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

