PML regulates apoptosis at endoplasmic reticulum by modulating calcium release
- PMID: 21030605
- PMCID: PMC3017677
- DOI: 10.1126/science.1189157
PML regulates apoptosis at endoplasmic reticulum by modulating calcium release
Erratum in
-
Erratum for the Report "PML Regulates Apoptosis at Endoplasmic Reticulum by Modulating Calcium Release" by C. Giorgi, K. Ito, H.-K. Lin, C. Santangelo, M. R. Wieckowski, M. Lebiedzinska, A. Bononi, M. Bonora, J. Duszynski, R. Bernardi, R. Rizzuto, C. Tacchetti, P. Pinton, P. P. Pandolfi.Science. 2021 Mar 26;371(6536):eabi4740. doi: 10.1126/science.abi4740. Science. 2021. PMID: 33766887 No abstract available.
Abstract
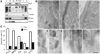
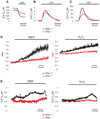
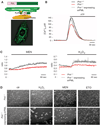
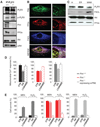
The promyelocytic leukemia (PML) tumor suppressor is a pleiotropic modulator of apoptosis. However, the molecular basis for such a diverse proapoptotic role is currently unknown. We show that extranuclear Pml was specifically enriched at the endoplasmic reticulum (ER) and at the mitochondria-associated membranes, signaling domains involved in ER-to-mitochondria calcium ion (Ca(2+)) transport and in induction of apoptosis. We found Pml in complexes of large molecular size with the inositol 1,4,5-trisphosphate receptor (IP(3)R), protein kinase Akt, and protein phosphatase 2a (PP2a). Pml was essential for Akt- and PP2a-dependent modulation of IP(3)R phosphorylation and in turn for IP(3)R-mediated Ca(2+) release from ER. Our findings provide a mechanistic explanation for the pleiotropic role of Pml in apoptosis and identify a pharmacological target for the modulation of Ca(2+) signals.
Figures
Comment in
-
Cell biology. Puzzled by PML.Science. 2010 Nov 26;330(6008):1183-4. doi: 10.1126/science.1199405. Science. 2010. PMID: 21109655 No abstract available.
-
Ca²(+) transfer from the ER to mitochondria: channeling cell death by a tumor suppressor.Dev Cell. 2010 Dec 14;19(6):789-90. doi: 10.1016/j.devcel.2010.11.013. Dev Cell. 2010. PMID: 21145493
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

