Physiological relevance of cell-specific distribution patterns of CFTR, NKCC1, NBCe1, and NHE3 along the crypt-villus axis in the intestine
- PMID: 21030607
- PMCID: PMC3025502
- DOI: 10.1152/ajpgi.00245.2010
Physiological relevance of cell-specific distribution patterns of CFTR, NKCC1, NBCe1, and NHE3 along the crypt-villus axis in the intestine
Abstract
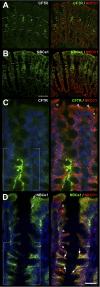
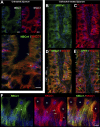
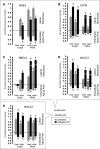
We examined the cell-specific subcellular expression patterns for sodium- and potassium-coupled chloride (NaK2Cl) cotransporter 1 (NKCC1), Na(+) bicarbonate cotransporter (NBCe1), cystic fibrosis transmembrane conductance regulator (CFTR), and Na(+)/H(+) exchanger 3 (NHE3) to understand the functional plasticity and synchronization of ion transport functions along the crypt-villus axis and its relevance to intestinal disease. In the unstimulated intestine, all small intestinal villus enterocytes coexpressed apical CFTR and NHE3, basolateral NBCe1, and mostly intracellular NKCC1. All (crypt and villus) goblet cells strongly expressed basolateral NKCC1 (at approximately three-fold higher levels than villus enterocytes), but no CFTR, NBCe1, or NHE3. Lower crypt cells coexpressed apical CFTR and basolateral NKCC1, but no NHE3 or NBCe1 (except NBCe1-expressing proximal colonic crypts). CFTR, NBCe1, and NKCC1 colocalized with markers of early and recycling endosomes, implicating endocytic recycling in cell-specific anion transport. Brunner's glands of the proximal duodenum coexpressed high levels of apical/subapical CFTR and basolateral NKCC1, but very low levels of NBCe1, consistent with secretion of Cl(-)-enriched fluid into the crypt. The cholinergic agonist carbachol rapidly (within 10 min) reduced cell volume along the entire crypt/villus axis and promoted NHE3 internalization into early endosomes. In contrast, carbachol induced membrane recruitment of NKCC1 and CFTR in all crypt and villus enterocytes, NKCC1 in all goblet cells, and NBCe1 in all villus enterocytes. These observations support regulated vesicle traffic in Cl(-) secretion by goblet cells and Cl(-) and HCO(3)(-) secretion by villus enterocytes during the transient phase of cholinergic stimulation. Overall, the carbachol-induced membrane trafficking profile of the four ion transporters supports functional plasticity of the small intestinal villus epithelium that enables it to conduct both absorptive and secretory functions.
Figures




References
-
- Ainsworth M, Koss MA, Hogan DL, Isenberg JI. Higher proximal duodenal mucosal bicarbonate secretion is independent of Brunner's glands in rats and rabbits. Gastroenterology 109: 1160–1166, 1995 - PubMed
-
- Allen A, Flemstrom G. Gastroduodenal mucus bicarbonate barrier: protection against acid and pepsin. Am J Physiol Cell Physiol 288: C1–C19, 2005 - PubMed
-
- Alper SL, Rossmann H, Wilhelm S, Stuart-Tilley AK, Shmukler BE, Seidler U. Expression of AE2 anion exchanger in mouse intestine. Am J Physiol Gastrointest Liver Physiol 277: G321–G332, 1999 - PubMed
-
- Ameen N, Alexis J, Salas P. Cellular localization of the cystic fibrosis transmembrane conductance regulator in mouse intestinal tract. Histochem Cell Biol 114: 69–75, 2000 - PubMed
-
- Ameen N, Apodaca G. Defective CFTR apical endocytosis and enterocyte brush border in myosin VI-deficient mice. Traffic 8: 998–1006, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

