Role of liver sinusoidal endothelial cells and stabilins in elimination of oxidized low-density lipoproteins
- PMID: 21030611
- PMCID: PMC3025507
- DOI: 10.1152/ajpgi.00215.2010
Role of liver sinusoidal endothelial cells and stabilins in elimination of oxidized low-density lipoproteins
Abstract
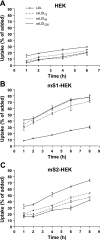
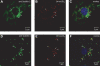
Atherogenesis is associated with elevated levels of low-density lipoprotein (LDL) and its oxidized form (oxLDL) in the blood. The liver is an important scavenger organ for circulating oxLDLs. The present study aimed to examine endocytosis of mildly oxLDL (the major circulating form of oxLDLs) in liver sinusoidal endothelial cells (LSECs) and the involvement of the scavenger receptors stabilin-1 and stabilin-2 in this process. Freshly isolated LSECs, Kupffer cells (KCs), and stabilin-1- and stabilin-2-transfected human embryonic kidney cells were incubated with fluorescently labeled or radiolabeled oxLDLs [oxidized for 3 h (oxLDL(3)), 6 h, or 24 h (oxLDL(24))] to measure endocytosis. The intracellular localization of oxLDLs and stabilins in LSECs was examined by immunofluorescence and immunogold electron microscopy. Whereas oxLDL(24) was endocytosed both by LSECs and KCs, oxLDL(3) (mildly oxLDL) was taken up by LSECs only. The LSEC uptake of oxLDLs was significantly inhibited by the scavenger receptor ligand formaldehyde-treated serum albumin. Uptake of all modified LDLs was high in stabilin-1-transfected cells, whereas stabilin-2-transfected cells preferentially took up oxLDL(24), suggesting that stabilin-1 is a more important receptor for mildly oxLDLs than stabilin-2. Double immunogold labeling experiments in LSECs indicated interactions of stabilin-1 and stabilin-2 with oxLDL(3) on the cell surface, in coated pits, and endocytic vesicles. LSECs but not KCs endocytosed mildly oxLDL. Both stabilin-1 and stabilin-2 were involved in the LSEC endocytosis of oxLDLs, but experiments with stabilin-transfected cells pointed to stabilin-1 as the most important receptor for mildly oxLDL.
Figures



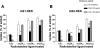


References
-
- Adachi H, Tsujimoto M. FEEL-1, a novel scavenger receptor with in vitro bacteria-binding and angiogenesis-modulating activities. J Biol Chem 277: 34264–34270, 2002 - PubMed
-
- Avogaro P, Bon GB, Cazzolato G. Presence of a modified low density lipoprotein in humans. Arteriosclerosis 8: 79–87, 1988 - PubMed
-
- Bourret G, Brodeur MR, Luangrath V, Lapointe J, Falstrault L, Brissette L. In vivo cholesteryl ester selective uptake of mildly and standardly oxidized LDL occurs by both parenchymal and nonparenchymal mouse hepatic cells but SR-BI is only responsible for standardly oxidized LDL selective uptake by nonparenchymal cells. Int J Biochem Cell Biol 38: 1160–1170, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

