Gene therapy-mediated delivery of targeted cytotoxins for glioma therapeutics
- PMID: 21030678
- PMCID: PMC2993419
- DOI: 10.1073/pnas.1008261107
Gene therapy-mediated delivery of targeted cytotoxins for glioma therapeutics
Abstract
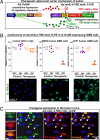
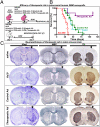
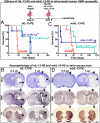
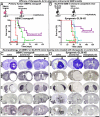
Restricting the cytotoxicity of anticancer agents by targeting receptors exclusively expressed on tumor cells is critical when treating infiltrative brain tumors such as glioblastoma multiforme (GBM). GBMs express an IL-13 receptor (IL13Rα2) that differs from the physiological IL4R/IL13R receptor. We developed a regulatable adenoviral vector (Ad.mhIL-4.TRE.mhIL-13-PE) encoding a mutated human IL-13 fused to Pseudomonas exotoxin (mhIL-13-PE) that specifically binds to IL13Rα2 to provide sustained expression, effective anti-GBM cytotoxicity, and minimal neurotoxicity. The therapeutic Ad also encodes mutated human IL-4 that binds to the physiological IL4R/IL13R without interacting with IL13Rα2, thus inhibiting potential binding of mhIL-13-PE to normal brain cells. Using intracranial GBM xenografts and syngeneic mouse models, we tested the Ad.mhIL-4.TRE.mhIL-13-PE and two protein formulations, hIL-13-PE used in clinical trials (Cintredekin Besudotox) and a second-generation mhIL-13-PE. Cintredekin Besudotox doubled median survival without eliciting long-term survival and caused severe neurotoxicity; mhIL-13-PE led to ∼40% long-term survival, eliciting severe neurological toxicity at the high dose tested. In contrast, Ad-mediated delivery of mhIL-13-PE led to tumor regression and long-term survival in over 70% of the animals, without causing apparent neurotoxicity. Although Cintredekin Besudotox was originally developed to target GBM, when tested in a phase III trial it failed to achieve clinical endpoints and revealed neurotoxicity. Limitations of Cintredekin Besudotox include its short half-life, which demanded frequent or continued administration, and binding to IL4R/IL13R, present in normal brain cells. These shortcomings were overcome by our therapeutic Ad, thus representing a significant advance in the development of targeted therapeutics for GBM.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The IL-4 and IL-13 pseudomonas exotoxins: new hope for brain tumor therapy.Neurosurg Focus. 2006 Apr 15;20(4):E11. doi: 10.3171/foc.2006.20.4.6. Neurosurg Focus. 2006. PMID: 16709016 Review.
-
Cintredekin besudotox in treatment of malignant glioma.Expert Opin Biol Ther. 2008 Jun;8(6):805-12. doi: 10.1517/14712598.8.6.805. Expert Opin Biol Ther. 2008. PMID: 18476792 Review.
-
Safety of intraparenchymal convection-enhanced delivery of cintredekin besudotox in early-phase studies.Neurosurg Focus. 2006 Apr 15;20(4):E15. Neurosurg Focus. 2006. PMID: 16709020 Clinical Trial.
-
Convection-enhanced delivery of interleukin-13 receptor-directed cytotoxin for malignant glioma therapy.Technol Cancer Res Treat. 2006 Jun;5(3):239-50. doi: 10.1177/153303460600500307. Technol Cancer Res Treat. 2006. PMID: 16700620 Review.
-
Novel anti-brain tumor cytotoxins specific for cancer cells.Nat Biotechnol. 1998 May;16(5):449-53. doi: 10.1038/nbt0598-449. Nat Biotechnol. 1998. PMID: 9592393
Cited by
-
New therapeutic approaches for malignant glioma: in search of the Rosetta stone.F1000 Med Rep. 2012;4:18. doi: 10.3410/M4-18. Epub 2012 Sep 5. F1000 Med Rep. 2012. PMID: 22991580 Free PMC article.
-
Gene therapy-mediated reprogramming tumor infiltrating T cells using IL-2 and inhibiting NF-κB signaling improves the efficacy of immunotherapy in a brain cancer model.Neurotherapeutics. 2012 Oct;9(4):827-43. doi: 10.1007/s13311-012-0144-7. Neurotherapeutics. 2012. PMID: 22996231 Free PMC article.
-
IL-13Rα2-Targeted Therapy Escapees: Biologic and Therapeutic Implications.Transl Oncol. 2011 Dec;4(6):390-400. doi: 10.1593/tlo.11175. Epub 2011 Dec 1. Transl Oncol. 2011. PMID: 22191003 Free PMC article.
-
Rodent Glioma Models: Intracranial Stereotactic Allografts and Xenografts.Neuromethods. 2012;77:229-243. doi: 10.1007/7657_2011_33. Epub 2012 Mar 13. Neuromethods. 2012. PMID: 31462854 Free PMC article.
-
Maximizing gene delivery efficiencies of cationic helical polypeptides via balanced membrane penetration and cellular targeting.Biomaterials. 2014 Jan;35(4):1302-14. doi: 10.1016/j.biomaterials.2013.09.090. Epub 2013 Nov 7. Biomaterials. 2014. PMID: 24211080 Free PMC article.
References
-
- Debinski W, Obiri NI, Powers SK, Pastan I, Puri RK. Human glioma cells overexpress receptors for interleukin 13 and are extremely sensitive to a novel chimeric protein composed of interleukin 13 and Pseudomonas exotoxin. Clin Cancer Res. 1995;1:1253–1258. - PubMed
-
- Husain SR, Joshi BH, Puri RK. Interleukin-13 receptor as a unique target for anti-glioblastoma therapy. Int J Cancer. 2001;92:168–175. - PubMed
-
- Joshi BH, Plautz GE, Puri RK. Interleukin-13 receptor α chain: A novel tumor-associated transmembrane protein in primary explants of human malignant gliomas. Cancer Res. 2000;60:1168–1172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

