A biochemical mechanism for the oncogenic potential of the p110beta catalytic subunit of phosphoinositide 3-kinase
- PMID: 21030680
- PMCID: PMC2993364
- DOI: 10.1073/pnas.1008739107
A biochemical mechanism for the oncogenic potential of the p110beta catalytic subunit of phosphoinositide 3-kinase
Abstract
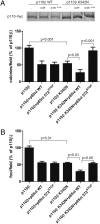
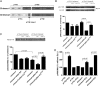
Class I PI3-kinases signal downstream of receptor tyrosine kinases and G protein-coupled receptors and have been implicated in tumorigenesis. Although the oncogenic potential of the PI3-kinase subunit p110α requires its mutational activation, other p110 isoforms can induce transformation when overexpressed in the wild-type state. In wild-type p110α, N345 in the C2 domain forms hydrogen bonds with D560 and N564 in the inter-SH2 (iSH2) domain of p85, and mutations of p110α or p85 that disrupt this interface lead to increased basal activity and transformation. Sequence analysis reveals that N345 in p110α aligns with K342 in p110β. This difference makes wild-type p110β analogous to a previously described oncogenic mutant, p110α-N345K. We now show that p110β is inhibited by p85 to a lesser extent than p110α and is not differentially inhibited by wild-type p85 versus p85 mutants that disrupt the C2-iSH2 domain interface. Similar results were seen in soft agar and focus-formation assays, where p110β was similar to p110α-N345K in transforming potential. Inhibition of p110β by p85 was enhanced by a K342N mutation in p110β, which led to decreased activity in vitro, decreased basal Akt and ribosomal protein S6 kinase (S6K1) activation, and decreased transformation in NIH 3T3 cells. Moreover, unlike wild-type p110β, p110β-K342N was differentially regulated by wild-type and mutant p85, suggesting that the inhibitory C2-iSH2 interface is functional in this mutant. This study shows that the enhanced transforming potential of p110β is the result of its decreased inhibition by p85, due to the disruption of an inhibitory C2-iSH2 domain interface.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. - PubMed
-
- Kurosu H, et al. Heterodimeric phosphoinositide 3-kinase consisting of p85 and p110beta is synergistically activated by the betagamma subunits of G proteins and phosphotyrosyl peptide. J Biol Chem. 1997;272:24252–24256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

