"Getting from here to there"--mechanisms and limitations to the activation of the androgen receptor in castration-resistant prostate cancer
- PMID: 21030877
- PMCID: PMC5589138
- DOI: 10.231/JIM.0b013e3181ff6bb8
"Getting from here to there"--mechanisms and limitations to the activation of the androgen receptor in castration-resistant prostate cancer
Abstract
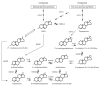
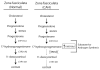
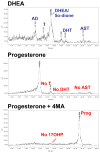
Despite the clinical regression that typifies the initial response of advanced prostate cancer to gonadal testosterone depletion, tumors eventually progress. However, evidence supports the concept that signaling via the androgen receptor (AR) is important in progression to castration-resistant prostate cancer (CRPC).Steroid hormones are synthesized from cholesterol in a series of tightly regulated steps involving the cleavage of carbon-carbon bonds, the introduction of functional groups derived from activated molecular oxygen, and the oxidation and reduction of carbon-carbon and carbon-oxygen bonds. In the adrenal cortex and gonads, steroidogenesis is tightly regulated, very efficient, and highly directional. In contrast, steroid metabolism in peripheral tissues is characterized by competing enzymes and pathways, low efficiency, and great variability. Many steps are mechanistically and functionally irreversible, but some are not, and the repertoire of specific enzymes, intracellular redox state, and access to hormone precursors all contribute to steroid flux and accumulation.The investigation of steroid metabolizing enzymes in CRPC often assumes that the pathways and the patterns of metabolism mirror those defined in the adrenals and the gonads and validated by human deficiency syndromes. Unfortunately, several potential pathways using different enzymes might contribute substantially to androgen synthesis in CRPC. Finally, a number of mechanisms have been reported by which the AR is activated independent of ligand. Recent observations have suggested that AR forms with constitutive activity occur in CRPC, stimulating transcription without a requirement for ligand. This overview outlines a broad view of how the mechanisms by which the AR may be activated, whether by alternate pathways of androgen synthesis or the production of alternate forms of the AR, with an emphasis on what aspects must be accounted for when using model systems to explore the biology of human prostate cancer.
Figures
Similar articles
-
Key targets of hormonal treatment of prostate cancer. Part 1: the androgen receptor and steroidogenic pathways.BJU Int. 2009 Aug;104(4):438-48. doi: 10.1111/j.1464-410X.2009.08695.x. Epub 2009 Jun 24. BJU Int. 2009. PMID: 19558559 Review.
-
New agents and strategies for the hormonal treatment of castration-resistant prostate cancer.Expert Opin Investig Drugs. 2010 Jul;19(7):837-46. doi: 10.1517/13543784.2010.494178. Expert Opin Investig Drugs. 2010. PMID: 20524793 Review.
-
Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth.Cancer Res. 2008 Jun 1;68(11):4447-54. doi: 10.1158/0008-5472.CAN-08-0249. Cancer Res. 2008. PMID: 18519708 Free PMC article.
-
Abiraterone switches castration-resistant prostate cancer dependency from adrenal androgens towards androgen receptor variants and glucocorticoid receptor signalling.Prostate. 2022 Apr;82(5):505-516. doi: 10.1002/pros.24297. Epub 2022 Jan 17. Prostate. 2022. PMID: 35037287 Free PMC article.
-
Dihydrotestosterone synthesis from adrenal precursors does not involve testosterone in castration-resistant prostate cancer.Cancer Biol Ther. 2012 Mar;13(5):237-8. doi: 10.4161/cbt.19608. Epub 2012 Mar 1. Cancer Biol Ther. 2012. PMID: 22336886 Free PMC article.
Cited by
-
The 5α-androstanedione pathway to dihydrotestosterone in castration-resistant prostate cancer.J Investig Med. 2012 Feb;60(2):504-7. doi: 10.2310/JIM.0b013e31823874a4. J Investig Med. 2012. PMID: 22064602 Free PMC article. Review.
-
Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer.Proc Natl Acad Sci U S A. 2011 Aug 16;108(33):13728-33. doi: 10.1073/pnas.1107898108. Epub 2011 Jul 27. Proc Natl Acad Sci U S A. 2011. PMID: 21795608 Free PMC article.
-
Minireview: Androgen metabolism in castration-resistant prostate cancer.Mol Endocrinol. 2013 May;27(5):708-14. doi: 10.1210/me.2013-1007. Epub 2013 Apr 16. Mol Endocrinol. 2013. PMID: 23592429 Free PMC article. Review.
-
Synthesis and Biological Evaluation of New Isoxazolyl Steroids as Anti-Prostate Cancer Agents.Int J Mol Sci. 2022 Nov 4;23(21):13534. doi: 10.3390/ijms232113534. Int J Mol Sci. 2022. PMID: 36362320 Free PMC article.
-
Role of single nucleotide polymorphisms of the HSD3B1 gene (rs6203 and rs33937873) in the prediction of prostate cancer risk.Mol Med Rep. 2022 Aug;26(2):271. doi: 10.3892/mmr.2022.12787. Epub 2022 Jul 7. Mol Med Rep. 2022. PMID: 35795973 Free PMC article.
References
-
- Simard J, Ricketts ML, Gingras S, Soucy P, Feltus FA, Melner MH. Molecular biology of the 3beta-hydroxysteroid dehydrogenase/delta5-delta4 isomerase gene family. Endocr Rev. 2005 Jun;26(4):525–582. - PubMed
-
- Auchus RJ. The backdoor pathway to dihydrotestosterone. Trends Endocrinol Metab. 2004 Nov;15(9):432–438. - PubMed
-
- Auchus RJ. Overview of dehydroepiandrosterone biosynthesis. Semin Reprod Med. 2004 Nov;22(4):281–288. - PubMed
-
- Andersson S, Geissler WM, Wu L, et al. Molecular genetics and pathophysiology of 17 beta-hydroxysteroid dehydrogenase 3 deficiency. J Clin Endocrinol Metab. 1996 Jan;81(1):130–136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

