Synthesis of oligodeoxynucleotides using fully protected deoxynucleoside 3'-phosphoramidite building blocks and base recognition of oligodeoxynucleotides incorporating N3-cyano-ethylthymine
- PMID: 21030906
- PMCID: PMC6259151
- DOI: 10.3390/molecules15117509
Synthesis of oligodeoxynucleotides using fully protected deoxynucleoside 3'-phosphoramidite building blocks and base recognition of oligodeoxynucleotides incorporating N3-cyano-ethylthymine
Abstract
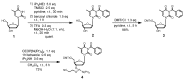
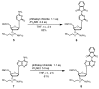
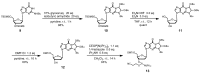
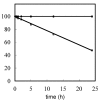
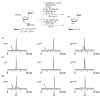
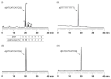
Oligodeoxynucleotide (ODN) synthesis, which avoids the formation of side products, is of great importance to biochemistry-based technology development. One side reaction of ODN synthesis is the cyanoethylation of the nucleobases. We suppressed this reaction by synthesizing ODNs using fully protected deoxynucleoside 3'-phosphoramidite building blocks, where the remaining reactive nucleobase residues were completely protected with acyl-, diacyl-, and acyl-oxyethylene-type groups. The detailed analysis of cyanoethylation at the nucleobase site showed that N3-protection of the thymine base efficiently suppressed the Michael addition of acrylonitrile. An ODN incorporating N3-cyanoethylthymine was synthesized using the phosphoramidite method, and primer extension reactions involving this ODN template were examined. As a result, the modified thymine produced has been proven to serve as a chain terminator.
Figures
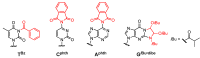

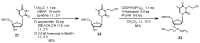

Similar articles
-
Convenient synthesis of N-unprotected deoxynucleoside 3'-phosphoramidite building blocks by selective deacylation of N-acylated species and their facile conversion to other N-functionalized derivatives.Org Lett. 2005 Nov 24;7(24):5389-92. doi: 10.1021/ol051949z. Org Lett. 2005. PMID: 16288513
-
Synthesis of 2'-deoxyoxanosine from 2'-deoxyguanosine, conversion to its phosphoramidite, and incorporation into oxanine-containing oligodeoxynucleotides.Curr Protoc Nucleic Acid Chem. 2010 Jun;Chapter 4:Unit 4.39. doi: 10.1002/0471142700.nc0439s41. Curr Protoc Nucleic Acid Chem. 2010. PMID: 20517989
-
Development of new N-unprotected phosphoramidite building blocks having a silyl-type linker.Nucleic Acids Symp Ser (Oxf). 2005;(49):127-8. doi: 10.1093/nass/49.1.127. Nucleic Acids Symp Ser (Oxf). 2005. PMID: 17150666
-
Dim and Dmoc Protecting Groups for Oligodeoxynucleotide Synthesis.Curr Protoc Nucleic Acid Chem. 2020 Sep;82(1):e111. doi: 10.1002/cpnc.111. Curr Protoc Nucleic Acid Chem. 2020. PMID: 32628352 Free PMC article.
-
Synthesis of 5'-O-phosphoramidites with a photolabile 3'-O-protecting group.Curr Protoc Nucleic Acid Chem. 2004 Sep;Chapter 12:Unit 12.3. doi: 10.1002/0471142700.nc1203s17. Curr Protoc Nucleic Acid Chem. 2004. PMID: 18428919 Review.
Cited by
-
Mini review: Enzyme-based DNA synthesis and selective retrieval for data storage.Comput Struct Biotechnol J. 2021 Apr 25;19:2468-2476. doi: 10.1016/j.csbj.2021.04.057. eCollection 2021. Comput Struct Biotechnol J. 2021. PMID: 34025937 Free PMC article. Review.
-
Design considerations for advancing data storage with synthetic DNA for long-term archiving.Mater Today Bio. 2022 May 27;15:100306. doi: 10.1016/j.mtbio.2022.100306. eCollection 2022 Jun. Mater Today Bio. 2022. PMID: 35677811 Free PMC article. Review.
References
-
- Ozsolak F., Platt A.R., Jones D.R., Reifenberger J.G. Sass, L.E., McInerney P., Thompson J.F., Bowers J., Jarosz M., Milos P.M. Direct RNA sequencing. Nature. 2009;461:814–819. - PubMed
-
- Rosi N.L., Giljohann D.A., Thaxton C.S., Lytton-Jean A.K.L., Han M.S., Mirkin C.A. Oligonucleotide-modified gold nanoparticles for intracellular gene reguration. Science. 2006;312:1027–1030. - PubMed
-
- Gibson D.G., Glass J.I., Lartigue C., Noskov V.N., Chuang R.Y., Algire M.A., Benders G.A., Montague M.G., Ma L., Moodie M.M., Merryman C., Vashee S., Krishnakumar R., Assad-Garcia N., Andrews-Pfannkoch C., Denisova E.A., Young L., Qi Z.Q., Segall-Shapiro T.H., Calvey C.H., Parmar P.P., Clyde A. Hutchison C.A., III, Smith H.O., Venter J.C. Creation of a Bacterial Cell Controlled by a Chemically Synthesized Genome. Science. 2010;329:52–56. - PubMed
-
- Gibson D.G., Benders G.A., Andrews-Pfannkoch C., Denisova E.A., Baden-Tillson H., Zaveri J., Stockwell T.B., Brownley A., Thomas D.W., Algire M.A., Merryman C., Young L., Noskov V.N., Glass J.I., Venter J.C., Hutchison C.A., III, Smith H.O. Complete Chemical Synthesis, Assembly, and Cloning of a Mycoplasma genitalium Genome. Science. 2008;319:1215–1220. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

