Synthesis and characterization of a Ru(II) complex with functionalized phenanthroline ligands having single-double linked anthracenyl and 1-methoxy-1-buten-3-yne moieties
- PMID: 21030910
- PMCID: PMC6259579
- DOI: 10.3390/molecules15117570
Synthesis and characterization of a Ru(II) complex with functionalized phenanthroline ligands having single-double linked anthracenyl and 1-methoxy-1-buten-3-yne moieties
Abstract
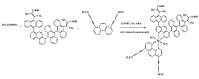
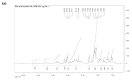
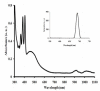
Two series of bidentate polypyridine ligands, made of phenanthroline chelating subunits having substituted mono-and di-anthracenyl groups, and 1-methoxy-1-buten-3-yne at the 4 and 7-positions with the corresponding heteroleptic Ru(II) complex have been synthesized and characterized. The complex is formulated as [(Ru(L(1))(L(2))(NCS)(2))], (where L(1 )= 4-(9-dianthracenyl-10-(2,3-dimethylacrylic acid)-7-(9-anthracenyl-10-(2,3-dimethylacrylic acid)-1,10-phenanthroline and L(2) = 4,7-bis(1-methoxy-1-buten-3-yne)-1,10-phenanthroline). The Ru(II) complex shows characteristic broad and intense metal-to-ligand charge transfer (MLCT) bands absorption and appreciable photoluminescence spanning the visible region. The ligands and complex were characterized by FT-IR, 1H, 13C NMR spectroscopy, UV-Vis, photoluminescence and elemental analysis (see in supplementary materials). The anchoring groups in both ligands have allowed an extended delocalization of acceptor orbital of the metal-to-ligand charge-transfer (MLCT) excited state.
Figures



Similar articles
-
Synthesis, photophysical and electrochemical properties of a mixed bipyridyl-phenanthrolyl ligand Ru(II) heteroleptic complex having trans-2-methyl-2-butenoic acid functionalities.Molecules. 2011 Sep 30;16(10):8353-67. doi: 10.3390/molecules16108353. Molecules. 2011. PMID: 21963625 Free PMC article.
-
A high molar extinction coefficient mono-anthracenyl bipyridyl heteroleptic ruthenium(II) complex: synthesis, photophysical and electrochemical properties.Molecules. 2011 Jun 3;16(6):4615-31. doi: 10.3390/molecules16064615. Molecules. 2011. PMID: 21642936 Free PMC article.
-
Synthesis and characterization of a heteroleptic Ru(II) complex of phenanthroline containing oligo-anthracenyl carboxylic acid moieties.Int J Mol Sci. 2010 Sep 8;11(9):3158-76. doi: 10.3390/ijms11093158. Int J Mol Sci. 2010. PMID: 20957086 Free PMC article.
-
Synthesis and photophysical and electrochemical properties of functionalized mono-, bis-, and trisanthracenyl bridged Ru(II) bis(2,2':6',2"-terpyridine) charge transfer complexes.ScientificWorldJournal. 2014;2014:570864. doi: 10.1155/2014/570864. Epub 2014 Apr 30. ScientificWorldJournal. 2014. PMID: 24883408 Free PMC article.
-
Mixed-ligand complexes of ruthenium(II) containing new photoactive or electroactive ligands: synthesis, spectral characterization and DNA interactions.J Biol Inorg Chem. 2005 Aug;10(5):496-508. doi: 10.1007/s00775-005-0660-6. Epub 2005 Sep 23. J Biol Inorg Chem. 2005. PMID: 15981005
Cited by
-
Synthesis, photophysical and electrochemical properties of a mixed bipyridyl-phenanthrolyl ligand Ru(II) heteroleptic complex having trans-2-methyl-2-butenoic acid functionalities.Molecules. 2011 Sep 30;16(10):8353-67. doi: 10.3390/molecules16108353. Molecules. 2011. PMID: 21963625 Free PMC article.
-
Synthesis and Assessment of Antibacterial Activities of Ruthenium(III) Mixed Ligand Complexes Containing 1,10-Phenanthroline and Guanide.Bioinorg Chem Appl. 2016;2016:3607924. doi: 10.1155/2016/3607924. Epub 2016 Oct 19. Bioinorg Chem Appl. 2016. PMID: 27833473 Free PMC article.
-
Towards the development of functionalized polypyridine ligands for Ru(II) complexes as photosensitizers in dye-sensitized solar cells (DSSCs).Molecules. 2014 Aug 15;19(8):12421-60. doi: 10.3390/molecules190812421. Molecules. 2014. PMID: 25153864 Free PMC article. Review.
-
A high molar extinction coefficient mono-anthracenyl bipyridyl heteroleptic ruthenium(II) complex: synthesis, photophysical and electrochemical properties.Molecules. 2011 Jun 3;16(6):4615-31. doi: 10.3390/molecules16064615. Molecules. 2011. PMID: 21642936 Free PMC article.
References
-
- Halpern J. Some aspects of the coordination and catalytic chemistry of ruthenium. Pure Appl. Chem. 1987;59:173–180. doi: 10.1351/pac198759020173. - DOI
-
- Juris A., Balzani V., Barigelletti F., Campagna S., Belser P., Zelewsky A.V. Ruthenium(II) polypyridine complexes: photophysics, photochemistry, electrochemistry, and chemiluminescence. Coord. Chem. Rev. 1988;84:85–277.
-
- Juris A., Campagna S., Balzani V., Gremaud G., Zelewsky A.V. Absorption spectra, luminescence properties, and electrochemical behaviour of tris-heteroleptic ruthenium(II) polypyridine complexes. Inorg. Chem. 1988:3652–3655.
-
- Prasanna de Silva A., Fox D.B., Moody T.S., Weir S.M. Luminescent sensors and photonic switches. Pure Appl. Chem. 2001;73:503–511. doi: 10.1351/pac200173030503. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

