The biosynthesis of artemisinin (Qinghaosu) and the phytochemistry of Artemisia annua L. (Qinghao)
- PMID: 21030913
- PMCID: PMC6259225
- DOI: 10.3390/molecules15117603
The biosynthesis of artemisinin (Qinghaosu) and the phytochemistry of Artemisia annua L. (Qinghao)
Abstract
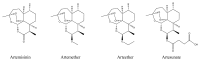
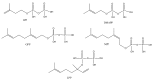
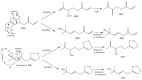
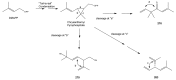
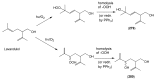
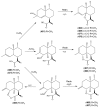
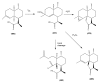
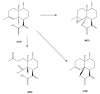
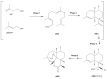
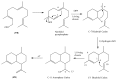
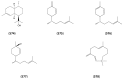
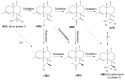
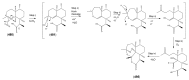
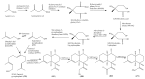
The Chinese medicinal plant Artemisia annua L. (Qinghao) is the only known source of the sesquiterpene artemisinin (Qinghaosu), which is used in the treatment of malaria. Artemisinin is a highly oxygenated sesquiterpene, containing a unique 1,2,4-trioxane ring structure, which is responsible for the antimalarial activity of this natural product. The phytochemistry of A. annua is dominated by both sesquiterpenoids and flavonoids, as is the case for many other plants in the Asteraceae family. However, A. annua is distinguished from the other members of the family both by the very large number of natural products which have been characterised to date (almost six hundred in total, including around fifty amorphane and cadinane sesquiterpenes), and by the highly oxygenated nature of many of the terpenoidal secondary metabolites. In addition, this species also contains an unusually large number of terpene allylic hydroperoxides and endoperoxides. This observation forms the basis of a proposal that the biogenesis of many of the highly oxygenated terpene metabolites from A. annua - including artemisinin itself - may proceed by spontaneous oxidation reactions of terpene precursors, which involve these highly reactive allyllic hydroperoxides as intermediates. Although several studies of the biosynthesis of artemisinin have been reported in the literature from the 1980s and early 1990s, the collective results from these studies were rather confusing because they implied that an unfeasibly large number of different sesquiterpenes could all function as direct precursors to artemisinin (and some of the experiments also appeared to contradict one another). As a result, the complete biosynthetic pathway to artemisinin could not be stated conclusively at the time. Fortunately, studies which have been published in the last decade are now providing a clearer picture of the biosynthetic pathways in A. annua. By synthesising some of the sesquiterpene natural products which have been proposed as biogenetic precursors to artemisinin in such a way that they incorporate a stable isotopic label, and then feeding these precursors to intact A. annua plants, it has now been possible to demonstrate that dihydroartemisinic acid is a late-stage precursor to artemisinin and that the closely related secondary metabolite, artemisinic acid, is not (this approach differs from all the previous studies, which used radio-isotopically labelled precursors that were fed to a plant homogenate or a cell-free preparation). Quite remarkably, feeding experiments with labeled dihydroartemisinic acid and artemisinic acid have resulted in incorporation of label into roughly half of all the amorphane and cadinane sesquiterpenes which were already known from phytochemical studies of A. annua. These findings strongly support the hypothesis that many of the highly oxygenated sesquiterpenoids from this species arise by oxidation reactions involving allylic hydroperoxides, which seem to be such a defining feature of the chemistry of A. annua. In the particular case of artemisinin, these in vivo results are also supported by in vitro studies, demonstrating explicitly that the biosynthesis of artemisinin proceeds via the tertiary allylic hydroperoxide, which is derived from oxidation of dihydroartemisinic acid. There is some evidence that the autoxidation of dihydroartemisinic acid to this tertiary allylic hydroperoxide is a non-enzymatic process within the plant, requiring only the presence of light; and, furthermore, that the series of spontaneous rearrangement reactions which then convert this allylic hydroperoxide to the 1,2,4-trioxane ring of artemisinin are also non-enzymatic in nature.
Figures
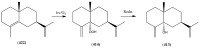
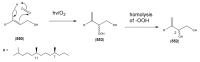
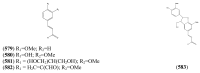
Similar articles
-
Identification of intermediates and enzymes involved in the early steps of artemisinin biosynthesis in Artemisia annua.Planta Med. 2005 Jan;71(1):40-7. doi: 10.1055/s-2005-837749. Planta Med. 2005. PMID: 15678372
-
Artemisia annua mutant impaired in artemisinin synthesis demonstrates importance of nonenzymatic conversion in terpenoid metabolism.Proc Natl Acad Sci U S A. 2016 Dec 27;113(52):15150-15155. doi: 10.1073/pnas.1611567113. Epub 2016 Dec 7. Proc Natl Acad Sci U S A. 2016. PMID: 27930305 Free PMC article.
-
Artemisinin and sesquiterpene precursors in dead and green leaves of Artemisia annua L. crops.Planta Med. 2007 Aug;73(10):1133-9. doi: 10.1055/s-2007-981567. Epub 2007 Jul 12. Planta Med. 2007. PMID: 17628838
-
Metabolic engineering of artemisinin biosynthesis in Artemisia annua L.Plant Cell Rep. 2011 May;30(5):689-94. doi: 10.1007/s00299-010-0967-9. Epub 2010 Dec 24. Plant Cell Rep. 2011. PMID: 21184232 Review.
-
Considerations on the mechanism of action of artemisinin antimalarials: part 1--the 'carbon radical' and 'heme' hypotheses.Infect Disord Drug Targets. 2013 Aug;13(4):217-77. doi: 10.2174/1871526513666131129155708. Infect Disord Drug Targets. 2013. PMID: 24304352 Review.
Cited by
-
Anti-inflammatory and acetylcholinesterase activity of extract, fractions and five compounds isolated from the leaves and twigs of Artemisia annua growing in Cameroon.Springerplus. 2016 Sep 9;5(1):1525. doi: 10.1186/s40064-016-3199-9. eCollection 2016. Springerplus. 2016. PMID: 27652098 Free PMC article.
-
An extract of the medicinal plant Artemisia annua modulates production of inflammatory markers in activated neutrophils.J Inflamm Res. 2015 Jan 14;8:9-14. doi: 10.2147/JIR.S75484. eCollection 2015. J Inflamm Res. 2015. PMID: 25609991 Free PMC article.
-
Multivariate Analysis of Essential Oil Composition of Artemisia annua L. Collected from Different Locations in Korea.Molecules. 2023 Jan 23;28(3):1131. doi: 10.3390/molecules28031131. Molecules. 2023. PMID: 36770797 Free PMC article.
-
Artemisinin-based antimalarial research: application of biotechnology to the production of artemisinin, its mode of action, and the mechanism of resistance of Plasmodium parasites.J Nat Med. 2016 Jul;70(3):318-34. doi: 10.1007/s11418-016-1008-y. Epub 2016 Jun 1. J Nat Med. 2016. PMID: 27250562 Free PMC article. Review.
-
Antimalarial properties and molecular docking analysis of compounds from Dioscorea bulbifera L. as new antimalarial agent candidates.BMC Complement Med Ther. 2021 May 18;21(1):144. doi: 10.1186/s12906-021-03317-y. BMC Complement Med Ther. 2021. PMID: 34006257 Free PMC article.
References
-
- Yeung H.C. Handbook of Chinese Herbs and Formulas. Volume 1. Institute of Chinese Medicine; Los Angeles, CA, USA: 1985. p. 430.
-
- . Pharmacopoeia of the People’s Republic of China, English edition ed. Guangdong Science and Technology Press; Guangzhou, China: 1992. p. 91.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

