Subtoxic levels hydrogen peroxide-induced production of interleukin-6 by retinal pigment epithelial cells
- PMID: 21031020
- PMCID: PMC2956668
Subtoxic levels hydrogen peroxide-induced production of interleukin-6 by retinal pigment epithelial cells
Abstract
Purpose: To study the effect of subtoxic levels of hydrogen peroxide (H(2)O(2)) on the expression and release of interleukin-6 (IL-6) by cultured retinal pigment epithelial (RPE) cells and to explore the relevant signal pathways.
Methods: Cultured human RPE cells were stimulated with various subtoxic concentrations of H(2)O(2) for different periods. Conditioned medium and cells were collected. IL-6 in the medium and IL-6 mRNA in the collected cells were measured using an IL-6 enzyme-linked immunosorbent assay kit and reverse transcriptase polymerase chain reaction, respectively. Nuclear factor-kappaB (NF-κB) in nuclear extracts and phosphorylated p38 mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase (ERK), and c-Jun N-terminal kinases (JNK) in cells cultured with and without H(2)O(2) were measured by NF-κB and MAPK enzyme-linked immunosorbent assay kits. Inhibitors of p38 (SB203580), ERK (UO1026), JNK (SP600125), and NF-κB (BAY11-7082) were added to the cultures before the addition of H(2)O(2) to test their effects(.)
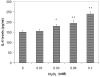
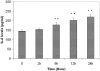
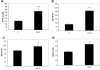
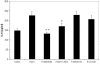
Results: Subtoxic levels of H(2)O(2) (100 µM and less) increased the IL-6 mRNA level and the release of IL-6 protein by the cultured human RPE cells in a dose- and time-dependent manner. This was accompanied by an increase of NF-κB in nuclear extracts and phosphorylated p38 MAPK, ERK, and JNK in cell lysates, particularly in the p38 and NF-κB. The NF-κB inhibitor decreased the H(2)O(2)-induced expression of IL-6. The p38 inhibitor, but not the ERK or JNK inhibitor, completely abolished H(2)O(2)-induced expression of IL-6 by RPE cells. The p38 inhibitor also abolished the increase of NF-κB in nuclear extracts in cells treated with H(2)O(2).
Conclusions: H(2)O(2) stimulated the production of IL-6, a key factor in the modulation of immune responses, inflammatory processes, and the occurrence of autoimmune diseases, which recently has been documented to be increased in age-related macular degeneration (AMD). This may be a molecular linkage for the oxidative stress and inflammatory/autoimmune reactions in AMD and may provide a novel target for the treatment of AMD.
Figures
References
-
- Rein DB, Wittenborn JS, Zhang X, Honeycutt AA, Lesesne SB, Saaddine J, Vision Health Cost-Effectiveness Study Group. Forecasting age-related macular degeneration through the year 2050: the potential impact of new treatments. Arch Ophthalmol. 2009;127:533–40. - PubMed
-
- Hogg R, Chakravarthy U. AMD and micronutrient antioxidants. Curr Eye Res. 2004;29:387–401. - PubMed
-
- Cai J, Nelson KC, Wu M, Sternberg P, Jr, Jones DP. Oxidative Damage and protection of the RPE. Prog Retin Eye Res. 2000;19:205–21. - PubMed
-
- Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol. 2004;122:598–614. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
