Disrupted modularity and local connectivity of brain functional networks in childhood-onset schizophrenia
- PMID: 21031030
- PMCID: PMC2965020
- DOI: 10.3389/fnsys.2010.00147
Disrupted modularity and local connectivity of brain functional networks in childhood-onset schizophrenia
Abstract
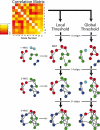
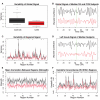
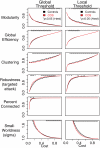
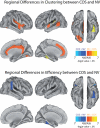
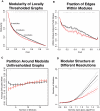
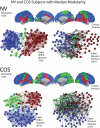
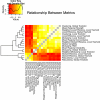
Modularity is a fundamental concept in systems neuroscience, referring to the formation of local cliques or modules of densely intra-connected nodes that are sparsely inter-connected with nodes in other modules. Topological modularity of brain functional networks can quantify theoretically anticipated abnormality of brain network community structure - so-called dysmodularity - in developmental disorders such as childhood-onset schizophrenia (COS). We used graph theory to investigate topology of networks derived from resting-state fMRI data on 13 COS patients and 19 healthy volunteers. We measured functional connectivity between each pair of 100 regional nodes, focusing on wavelet correlation in the frequency interval 0.05-0.1 Hz, then applied global and local thresholding rules to construct graphs from each individual association matrix over the full range of possible connection densities. We show how local thresholding based on the minimum spanning tree facilitates group comparisons of networks by forcing the connectedness of sparse graphs. Threshold-dependent graph theoretical results are compatible with the results of a k-means unsupervised learning algorithm and a multi-resolution (spin glass) approach to modularity, both of which also find community structure but do not require thresholding of the association matrix. In general modularity of brain functional networks was significantly reduced in COS, due to a relatively reduced density of intra-modular connections between neighboring regions. Other network measures of local organization such as clustering were also decreased, while complementary measures of global efficiency and robustness were increased, in the COS group. The group differences in complex network properties were mirrored by differences in simpler statistical properties of the data, such as the variability of the global time series and the internal homogeneity of the time series within anatomical regions of interest.
Keywords: brain; clustering; fMRI; graph theory; modularity; network; schizophrenia.
Figures

References
-
- Anderson B., Butts C., Carley K. (1999). The interaction of size and density with graph-level indices. Soc. Networks 21, 239–267 10.1016/S0378-8733(99)00011-8 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

