Reinforcement of a minor alternative splicing event in MYO7A due to a missense mutation results in a mild form of retinopathy and deafness
- PMID: 21031134
- PMCID: PMC2956701
Reinforcement of a minor alternative splicing event in MYO7A due to a missense mutation results in a mild form of retinopathy and deafness
Abstract
Purpose: Recessive mutations of the myosin VIIA (MYO7A) gene are reported to be responsible for both a deaf-blindness syndrome (Usher type 1B [USH1B] and atypical Usher syndrome) and nonsyndromic hearing loss (HL; Deafness, Neurosensory, Autosomal Recessive 2 [DFNB2]). The existence of DFNB2 is controversial, and often there is no relationship between the type and location of the MYO7A mutations corresponding to the USH1B and DFNB2 phenotype. We investigated the molecular determinant of a mild form of retinopathy in association with a subtle splicing modulation of MYO7A mRNA.
Methods: Affected members underwent detailed audiologic and ocular characterization. DNA samples from family members were genotyped with polymorphic microsatellite markers. Sequencing of MYO7A was performed. Endogenous lymphoid RNA analysis and a splicing minigene assay were used to study the effect of the c.1935G>A mutation.
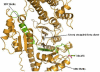
Results: Funduscopy showed mild retinitis pigmentosa in adults with HL. Microsatellite analysis showed linkage to markers in the region on chromosome 11q13.5. Sequencing of MYO7A revealed a mutation in the last nucleotide of exon 16 (c.1935G>A), which corresponds to a substitution of a methionine to an isoleucine residue at amino acid 645 of the myosin VIIA. However, structural prediction of the molecular model of myosin VIIA shows that this amino acid replacement induces only minor structural changes in the immediate environment of the mutation and thus does not alter the overall native structure. We found that, although predominantly included in mature mRNA, exon 16 is in fact alternatively spliced in control cells and that the mutation at the very last position is associated with a switch toward a predominant exclusion of that exon. This observation was further supported using a splicing minigene transfection assay; the c.1935G>A mutation was found to trigger a partial impairment of the adjacent donor splice site, suggesting that the unique change at the last position of the exon is responsible for the enhanced exon exclusion in this family.
Conclusions: This study shows how an exonic mutation that weakens the 5' splice site enhances a minor alternative splicing without abolishing a complete exclusion of the exon and therefore causes a less severe retinitis pigmentosa than the USH1B-associated alleles. It would be interesting to examine a possible correlation between intrafamilial phenotypic variability and the subtle variation in exon 16 inclusion, probably related to genetic background specificities.
Figures


Similar articles
-
Mutation spectrum of MYO7A and evaluation of a novel nonsyndromic deafness DFNB2 allele with residual function.Hum Mutat. 2008 Apr;29(4):502-11. doi: 10.1002/humu.20677. Hum Mutat. 2008. PMID: 18181211
-
The autosomal recessive isolated deafness, DFNB2, and the Usher 1B syndrome are allelic defects of the myosin-VIIA gene.Nat Genet. 1997 Jun;16(2):191-3. doi: 10.1038/ng0697-191. Nat Genet. 1997. PMID: 9171833
-
Novel compound heterozygous synonymous and missense variants in the MYO7A gene identified by next-generation sequencing in a Chinese family with nonsyndromic hearing loss.J Clin Lab Anal. 2022 Nov;36(11):e24708. doi: 10.1002/jcla.24708. Epub 2022 Sep 26. J Clin Lab Anal. 2022. PMID: 36164746 Free PMC article.
-
Unconventional myosins and the genetics of hearing loss.Am J Med Genet. 1999 Sep 24;89(3):147-57. doi: 10.1002/(sici)1096-8628(19990924)89:3<147::aid-ajmg5>3.0.co;2-6. Am J Med Genet. 1999. PMID: 10704189 Review.
-
The many different cellular functions of MYO7A in the retina.Biochem Soc Trans. 2011 Oct;39(5):1207-10. doi: 10.1042/BST0391207. Biochem Soc Trans. 2011. PMID: 21936790 Free PMC article. Review.
Cited by
-
Atypical and ultra-rare Usher syndrome: a review.Ophthalmic Genet. 2020 Oct;41(5):401-412. doi: 10.1080/13816810.2020.1747090. Epub 2020 May 6. Ophthalmic Genet. 2020. PMID: 32372680 Free PMC article. Review.
-
Genetics and genomic medicine in Tunisia.Mol Genet Genomic Med. 2018 Mar;6(2):134-159. doi: 10.1002/mgg3.392. Mol Genet Genomic Med. 2018. PMID: 29663716 Free PMC article.
-
Whole exome sequencing, in silico and functional studies confirm the association of the GJB2 mutation p.Cys169Tyr with deafness and suggest a role for the TMEM59 gene in the hearing process.Saudi J Biol Sci. 2021 Aug;28(8):4421-4429. doi: 10.1016/j.sjbs.2021.04.036. Epub 2021 Apr 20. Saudi J Biol Sci. 2021. PMID: 34354426 Free PMC article.
-
Inherited Retinal Disease Therapies Targeting Precursor Messenger Ribonucleic Acid.Vision (Basel). 2017 Sep 1;1(3):22. doi: 10.3390/vision1030022. Vision (Basel). 2017. PMID: 31740647 Free PMC article. Review.
-
Phenotype of Usher syndrome type II assosiated with compound missense mutations of c.721 C>T and c.1969 C>T in MYO7A in a Chinese Usher syndrome family.Int J Ophthalmol. 2015 Aug 18;8(4):670-4. doi: 10.3980/j.issn.2222-3959.2015.04.05. eCollection 2015. Int J Ophthalmol. 2015. PMID: 26309859 Free PMC article.
References
-
- Self T, Mahony M, Fleming J, Walsh J, Brown SD, Steel KP. Shaker-1 mutations reveal roles for myosin VIIA in both development and function of cochlear hair cells. Development. 1998;125:557–66. - PubMed
-
- Kros CJ, Marcotti W, van Netten SM, Self TJ, Libby RT, Brown SD, Richardson GP, Steel KP. Reduced climbing and increased slipping adaptation in cochlear hair cells of mice with Myo7a mutations. Nat Neurosci. 2002;5:41–7. - PubMed
-
- Wolfrum U, Liu X, Schmitt A, Udovichenko IP, Williams DS. Myosin VIIa as a common component of cilia and microvilli. Cell Motil Cytoskeleton. 1998;40:261–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
