Solvation of Biomolecules by the Soft Sticky Dipole-Quadrupole-Octupole Water Model
- PMID: 21031143
- PMCID: PMC2963461
- DOI: 10.1016/j.cplett.2009.12.089
Solvation of Biomolecules by the Soft Sticky Dipole-Quadrupole-Octupole Water Model
Abstract
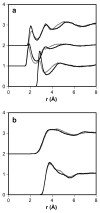
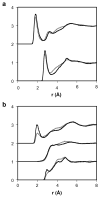
The soft sticky dipole-quadrupole-octupole (SSDQO) potential energy function represents a water molecule by a single site with a van der Waals sphere and point multipoles. Previously, SSDQO was shown to give good properties for liquid water and solvation of simple ions and is faster than three point models. Here, SSDQO is assessed for solvating biologically relevant molecules having a multi-site, partial charge description. Monte Carlo simulations of ethanol, benzene, and N-methylacetamide in SSDQO with SPC/E moments showed the water structure was as good as in SPC/E. Thus, SSDQO is potentially useful for simulations of biological macromolecules in aqueous solution.
Figures


References
-
- Berendsen HJC, Grigera JR, Straatsma TP. J Phys Chem. 1987;91:6269.
-
- Jorgensen WL. J Am Chem Soc. 1981;103:335.
-
- van der Spoel D, van Maaren PJ, Berendsen HJC. J Chem Phys. 1998;108:10220.
-
- Mahoney MW, Jorgensen WL. J Chem Phys. 2000;112:8910.
-
- Ichiye T, Tan ML. J Chem Phys. 2006;124:134504. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
