Dynamics for Reactions of Ion Pairs in Aqueous Solution: Reactivity of Tosylate Anion Ion Paired with the Highly Destabilized 1-(4-Methylphenyl)-2,2,2-Trifluoroethyl Carbocation
- PMID: 21031146
- PMCID: PMC2963475
- DOI: 10.1002/poc.1642
Dynamics for Reactions of Ion Pairs in Aqueous Solution: Reactivity of Tosylate Anion Ion Paired with the Highly Destabilized 1-(4-Methylphenyl)-2,2,2-Trifluoroethyl Carbocation
Abstract
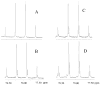
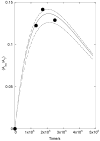
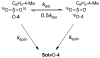
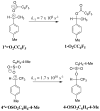
The sum of the rate constants for solvolysis and scrambling of carbon bridging and nonbridging oxygen-18 at 4-MeC(6)H(4)CH(CF(3))OS((18)O(2))Tos in 50/50 (v/v) trifluoroethanol/water, (k(solv) + k(iso)) = 5.4 × 10(-6) s(-1), is 50% larger than k(solv) = 3.6 × 10(-6) for the simple solvolysis reaction of the sulfonate ester. This shows that the ion pair intermediate of solvolysis undergoes significant internal return to form reactant. These data give a value of k(-1) = 1.7 × 10(10) s(-1) for internal return of the carbocation-anion pair to the substrate. This rate constant is larger than the value of k(-1) = 7 × 10(9) s(-1) reported for internal return of an ion pair between the 1-(4-methylphenyl)ethyl carbocation and pentafluorobenzoate anion to the neutral ester (4-MeC(6)H(4)CH(CH(3))O(2)CC(6)F(5)) in the same solvent. The partitioning of ion pairs to the 1-(4-methylphenyl)ethyl carbocation and to the highly destabilized 1-(4-methylphenyl)2,2,2-trifluoroethyl carbocation is compared and contrasted.
Figures
Similar articles
-
Dynamics for reaction of an ion pair in aqueous solution: reactivity of carboxylate anions in bimolecular carbocation-nucleophile addition and unimolecular ion pair collapse.Org Lett. 2001 Apr 19;3(8):1237-40. doi: 10.1021/ol015706s. Org Lett. 2001. PMID: 11348203
-
Reactions of ion-pair intermediates of solvolysis.Chem Rec. 2005;5(2):94-106. doi: 10.1002/tcr.20038. Chem Rec. 2005. PMID: 15825158
-
3-(Trifluoromethyl)indenyl Cation: Ion Pair Return in the Formation of an Antiaromatic and Electron-Deficient Doubly Destabilized Carbocation.J Org Chem. 1997 Jan 24;62(2):246-252. doi: 10.1021/jo961387k. J Org Chem. 1997. PMID: 11671396
-
Scrambling of oxygen-18 during the "borderline" solvolysis of 1-(3-nitrophenyl)ethyl tosylate.Org Lett. 2004 Sep 30;6(20):3633-6. doi: 10.1021/ol0484409. Org Lett. 2004. PMID: 15387566
-
The use of isotope effects to determine enzyme mechanisms.Arch Biochem Biophys. 2005 Jan 1;433(1):2-12. doi: 10.1016/j.abb.2004.08.027. Arch Biochem Biophys. 2005. PMID: 15581561 Review.
Cited by
-
Swain-Scott Relationships for Nucleophile Addition to Ring-Substituted Phenonium Ions.Can J Chem. 2015;93(4):428-434. doi: 10.1139/cjc-2014-0337. Epub 2014 Sep 8. Can J Chem. 2015. PMID: 26843657 Free PMC article.
-
Substituent Effects on the Formation and Nucleophile Selectivity of Ring-Substituted Phenonium Ions in Aqueous Solution.J Phys Org Chem. 2013 Dec 1;26(12):970-976. doi: 10.1002/poc.3145. J Phys Org Chem. 2013. PMID: 24511183 Free PMC article.
-
Formation and Mechanism for Reactions of Ring-Substituted Phenonium Ions in Aqueous Solution.J Phys Org Chem. 2016 Nov;29(11):557-564. doi: 10.1002/poc.3510. Epub 2015 Nov 30. J Phys Org Chem. 2016. PMID: 27812239 Free PMC article.
References
-
- Harris JM. Prog Phys Org Chem. 1974;11:89–173.
-
- Raber DJ, Harris JM, Schleyer PvR. In: Ions and Ion Pairs in Organic Reactions. Szwarc M, editor. John Wiley & Son; New York: 1974. p. 2.
-
- Tsuji Y, Kim SH, Saeki Y, Yatsugi KI, Fujio M, Tsuno Y. Tetrahedron Lett. 1995;36:1465–8.
-
- Allen AD, Fujio M, Tee OS, Tidwell TT, Tsuji Y, Tsuno Y, Yatsugi KI. J Am Chem Soc. 1995;117:8974–8981.
-
- Tsuji Y, Mori T, Toteva MM, Richard JP. J Phys Org Chem. 2003;16:484–490.
Grants and funding
LinkOut - more resources
Full Text Sources
