Microglia in close vicinity of glioma cells: correlation between phenotype and metabolic alterations
- PMID: 21031160
- PMCID: PMC2965014
- DOI: 10.3389/fnene.2010.00131
Microglia in close vicinity of glioma cells: correlation between phenotype and metabolic alterations
Abstract
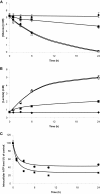
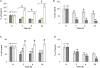
Microglia are immune cells within the central nervous system. In brain-developing tumors, gliomas are able to silence the defense and immune functions of microglia, a phenomenon which strongly contributes to tumor progression and treatment resistance. Being activated and highly motile, microglia infiltrate tumors and secrete macrophagic chemoattractant factors. Thereafter, the tumor cells shut down their immune properties and stimulate the microglia to release tumor growth-promoting factors. The result of such modulation is that a kind of symbiosis occurs between microglia and tumor cells, in favor of tumor growth. However, little is known about microglial phenotype and metabolic modifications in a tumoral environment. Co-cultures were performed using CHME5 microglia cells grown on collagen beads or on coverslips and placed on monolayer of C6 cells, limiting cell/cell contacts. Phagocytic behavior and expression of macrophagic and cytoskeleton markers were monitored. Respiratory properties and energetic metabolism were also studied with regard to the activated phenotype of microglia. In co-cultures, transitory modifications of microglial morphology and metabolism were observed linked to a concomitant transitory increase of phagocytic properties. Therefore, after 1 h of co-culture, microglia were activated but when longer in contact with tumor cells, phagocytic properties appear silenced. Like the behavior of the phenotype, microglial respiration showed a transitory readjustment although the mitochondria maintained their perinuclear relocation. Nevertheless, the energetic metabolism of the microglia was altered, suggesting a new energetic steady state. The results clearly indicate that like the depressed immune properties, the macrophagic and metabolic status of the microglia is quickly driven by the glioma environment, despite short initial phagocytic activation. Such findings question the possible contribution of diffusible tumor factors to the microglial metabolism.
Keywords: C6 cells; NMR spectroscopy; glioma; metabolism; microglia; phenotype.
Figures
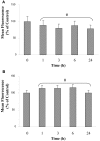

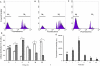


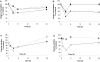
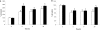
References
-
- Aloisi F. (2001). Immune function of microglia. Glia 36, 165–179 - PubMed
-
- Asai J., Suzuki R., Fujimoto T., Suzuki T., Nakagawa N., Nagashima G., Miyo T., Hokaku H., Takei A. (1999). Fluorescence automatic cell sorter and immunohistochemical investigation of CD68-positive cells in meningioma. Clin. Neurol. Neurosurg. 101, 229–234 10.1016/S0303-8467(99)00052-9 - DOI - PubMed
LinkOut - more resources
Full Text Sources

