The rise and fall of Dimebon
- PMID: 21031168
- PMCID: PMC3922928
- DOI: 10.1358/dnp.2010.23.8.1500435
The rise and fall of Dimebon
Abstract
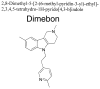
Dimebon (latrepirdine) was developed and used in Russia as an over-the-counter oral antihistamine for allergy treatment. In the early 1990s, Dimebon was characterized as a low-affinity NMDA receptor antagonist by Dr. Sergey Bachurin and his colleagues. An initial small-scale, open-label trial of Dimebon in 14 Alzheimer's disease (AD) patients demonstrated potential efficacy. Dimebon was then patented for the treatment of neurodegenerative disorders and licensed by Medivation. Extremely promising results were obtained in a double-blind, placebo-controlled, phase II AD trial in 183 patients; however, a phase II trial of Dimebon in 91 Huntington's disease patients was much less successful. Recently, a phase III AD trial of Dimebon in 598 patients failed to result in any significant improvement in primary or secondary outcomes. The failure of Dimebon may be in large part due to insufficient understanding of its mechanism of action. The NMDA receptor blocking activity of Dimebon is too weak to be physiologically relevant, while the proposed "novel mitochondrial mechanism of action" lacks credible scientific evidence or a molecular target. Independent studies indicate that the clinical effects of Dimebon most likely result from inhibition of histamine H₁ and serotonin 5-HT₆ receptors. Careful preclinical studies of novel potential therapies are needed to minimize chances of making similar costly mistakes in the future.
Copyright 2010 Prous Science, S.A.U. or its licensors. All rights reserved.
Figures

References
-
- Matveeva IA. Action of dimebon on histamine receptors. Farmakol Toksikol. 1983;46:27–9. - PubMed
-
- Zefirov NS, Afanasiev AZ, Afanasieva SV, Bachurin SO, Tkachenko SE, Grigoriev VV, Jurovskaya MA, Chetverikov VP, Bukatina EE, Grigorieva IV. Agents for treating neurodegenerative disorders. 7,071,206. US Patent. 2006 Jul 4;
-
- Bachurin S, Bukatina E, Lermontova N, Tkachenko S, Afanasiev A, Grigoriev V, Grigorieva I, Ivanov Y, Sablin S, Zefirov N. Antihistamine agent Dimebon as a novel neuroprotector and a cognition enhancer. Ann N Y Acad Sci. 2001;939:425–35. - PubMed
-
- Lermontova NN, Lukoyanov NV, Serkova TP, Lukoyanova EA, Bachurin SO. Dimebon improves learning in animals with experimental Alzheimer’s disease. Bull Exp Biol Med. 2000;129:544–6. - PubMed
-
- Doody RS, Gavrilova SI, Sano M, Thomas RG, Aisen PS, Bachurin SO, Seely L, Hung D. Effect of dimebon on cognition, activities of daily living, behaviour, and global function in patients with mild-to-moderate Alzheimer’s disease: a randomised, double-blind, placebo-controlled study. Lancet. 2008;372:207–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
