Far from solved: a perspective on what we know about early mechanisms of left-right asymmetry
- PMID: 21031419
- PMCID: PMC10468760
- DOI: 10.1002/dvdy.22450
Far from solved: a perspective on what we know about early mechanisms of left-right asymmetry
Abstract
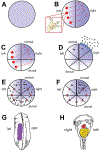
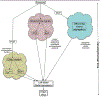
Consistent laterality is a crucial aspect of embryonic development, physiology, and behavior. While strides have been made in understanding unilaterally expressed genes and the asymmetries of organogenesis, early mechanisms are still poorly understood. One popular model centers on the structure and function of motile cilia and subsequent chiral extracellular fluid flow during gastrulation. Alternative models focus on intracellular roles of the cytoskeleton in driving asymmetries of physiological signals or asymmetric chromatid segregation, at much earlier stages. All three models trace the origin of asymmetry back to the chirality of cytoskeletal organizing centers, but significant controversy exists about how this intracellular chirality is amplified onto cell fields. Analysis of specific predictions of each model and crucial recent data on new mutants suggest that ciliary function may not be a broadly conserved, initiating event in left-right patterning. Many questions about embryonic left-right asymmetry remain open, offering fascinating avenues for further research in cell, developmental, and evolutionary biology.
© 2010 Wiley-Liss, Inc.
Figures
Similar articles
-
A unified model for left-right asymmetry? Comparison and synthesis of molecular models of embryonic laterality.Dev Biol. 2013 Jul 1;379(1):1-15. doi: 10.1016/j.ydbio.2013.03.021. Epub 2013 Apr 10. Dev Biol. 2013. PMID: 23583583 Free PMC article. Review.
-
The embryonic origins of left-right asymmetry.Crit Rev Oral Biol Med. 2004 Jul 1;15(4):197-206. doi: 10.1177/154411130401500403. Crit Rev Oral Biol Med. 2004. PMID: 15284185 Review.
-
Localization and loss-of-function implicates ciliary proteins in early, cytoplasmic roles in left-right asymmetry.Dev Dyn. 2005 Sep;234(1):176-89. doi: 10.1002/dvdy.20509. Dev Dyn. 2005. PMID: 16059906
-
Conserved roles for cytoskeletal components in determining laterality.Integr Biol (Camb). 2016 Mar 14;8(3):267-86. doi: 10.1039/c5ib00281h. Integr Biol (Camb). 2016. PMID: 26928161 Free PMC article. Review.
-
Perspectives and open problems in the early phases of left-right patterning.Semin Cell Dev Biol. 2009 Jun;20(4):456-63. doi: 10.1016/j.semcdb.2008.11.010. Epub 2008 Nov 25. Semin Cell Dev Biol. 2009. PMID: 19084609 Free PMC article. Review.
Cited by
-
Polysplenia syndrome: a review of the relationship with viscero-atrial situs and the spectrum of extra-cardiac anomalies.Surg Radiol Anat. 2013 Oct;35(8):647-53. doi: 10.1007/s00276-013-1100-x. Epub 2013 Mar 19. Surg Radiol Anat. 2013. PMID: 23508931 Review.
-
Conflict between Intrinsic Leaf Asymmetry and Phyllotaxis in the Resupinate Leaves of Alstroemeria psittacina.Front Plant Sci. 2012 Aug 10;3:182. doi: 10.3389/fpls.2012.00182. eCollection 2012. Front Plant Sci. 2012. PMID: 22908025 Free PMC article.
-
Early, nonciliary role for microtubule proteins in left-right patterning is conserved across kingdoms.Proc Natl Acad Sci U S A. 2012 Jul 31;109(31):12586-91. doi: 10.1073/pnas.1202659109. Epub 2012 Jul 16. Proc Natl Acad Sci U S A. 2012. PMID: 22802643 Free PMC article.
-
Neurally Derived Tissues in Xenopus laevis Embryos Exhibit a Consistent Bioelectrical Left-Right Asymmetry.Stem Cells Int. 2012;2012:353491. doi: 10.1155/2012/353491. Epub 2012 Dec 30. Stem Cells Int. 2012. PMID: 23346115 Free PMC article.
-
Reciprocal signaling between the ectoderm and a mesendodermal left-right organizer directs left-right determination in the sea urchin embryo.PLoS Genet. 2012;8(12):e1003121. doi: 10.1371/journal.pgen.1003121. Epub 2012 Dec 13. PLoS Genet. 2012. PMID: 23271979 Free PMC article.
References
-
- Abe T, Thitamadee S, Hashimoto T. 2004. Microtubule defects and cell morphogenesis in the lefty1lefty2 tubulin mutant of Arabidopsis thaliana. Plant Cell Physiol 45:211–220. - PubMed
-
- Adams DS, Levin M. 2006b.Strategies and techniques for investigation of biophysical signals in patterning. In:Whitman M,Sater AK, editors. Analysis of growth factor signaling in embryos. London: Taylor and Francis Books. p 177–262.
-
- Afzelius BA, Stenram U. 2006. Prevalence and genetics of immotile-cilia syndrome and left-handedness. Int J Dev Biol 50:571–573. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

