Real-time diffusion-perfusion mismatch analysis in acute stroke
- PMID: 21031505
- PMCID: PMC2975404
- DOI: 10.1002/jmri.22338
Real-time diffusion-perfusion mismatch analysis in acute stroke
Abstract
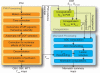
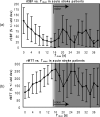
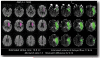
Diffusion-perfusion mismatch can be used to identify acute stroke patients that could benefit from reperfusion therapies. Early assessment of the mismatch facilitates necessary diagnosis and treatment decisions in acute stroke. We developed the RApid processing of PerfusIon and Diffusion (RAPID) for unsupervised, fully automated processing of perfusion and diffusion data for the purpose of expedited routine clinical assessment. The RAPID system computes quantitative perfusion maps (cerebral blood volume, CBV; cerebral blood flow, CBF; mean transit time, MTT; and the time until the residue function reaches its peak, T(max)) using deconvolution of tissue and arterial signals. Diffusion-weighted imaging/perfusion-weighted imaging (DWI/PWI) mismatch is automatically determined using infarct core segmentation of ADC maps and perfusion deficits segmented from T(max) maps. The performance of RAPID was evaluated on 63 acute stroke cases, in which diffusion and perfusion lesion volumes were outlined by both a human reader and the RAPID system. The correlation of outlined lesion volumes obtained from both methods was r(2) = 0.99 for DWI and r(2) = 0.96 for PWI. For mismatch identification, RAPID showed 100% sensitivity and 91% specificity. The mismatch information is made available on the hospital's PACS within 5-7 min. Results indicate that the automated system is sufficiently accurate and fast enough to be used for routine care as well as in clinical trials.
© 2010 Wiley-Liss, Inc.
Figures






References
-
- Albers GW, Thijs VN, Wechsler L, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006;60:508–517. - PubMed
-
- Davis SM, Donnan GA, Parsons MW, et al. Effects of alteplase beyond 3 h after stroke in the echoplanar imaging thrombolytic evaluation trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008;7:299–309. - PubMed
-
- Østergaard L, Weisskoff RM, Chesler DA, Gyldensted C, Rosen BR. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: Mathematical approach and statistical analysis. Magn Reson Med. 1996;36:715–725. - PubMed
-
- Mouridsen K, Christensen S, Gyldensted L, Ostergaard L. Automatic selection of arterial input function using cluster analysis. Magn Reson Med. 2006;55:524–531. - PubMed
-
- Kosior JC, Frayne R. PerfTool: a software platform for investigating bolus-tracking perfusion imaging quantification strategies. J Magn Reson Imag. 2007;25:653–659. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

