Alternative splicing of neuroligin and its protein distribution in the outer plexiform layer of the chicken retina
- PMID: 21031560
- PMCID: PMC4065168
- DOI: 10.1002/cne.22499
Alternative splicing of neuroligin and its protein distribution in the outer plexiform layer of the chicken retina
Abstract
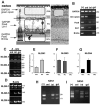
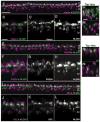
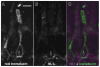
Although synaptogenesis within the retina is obviously essential for vision, mechanisms responsible for the initiation and maintenance of retinal synapses are poorly understood. In addition to its scientific interest, understanding retinal synapse formation is becoming clinically relevant with ongoing efforts to develop transplantation-based approaches for the treatment of retinal degenerative disease. To extend our understanding, we have focused on the chick model system and have studied the neuroligin family of neuronal adhesion factors that has been shown to participate in synapse assembly in the brain. We identified chicken orthologs of neuroligins 1, -3, and -4, but could find no evidence of neuroligin 2. We investigated temporal and spatial patterns of mRNA and protein expression during development using standard polymerase chain reaction (RT-PCR), quantitative PCR (QPCR), laser-capture microdissection (LCM), and confocal microscopy. At the mRNA level, neuroligins were detected at the earliest period tested, embryonic day (ED)5, which precedes the period of inner retina synaptogenesis. Significant alternative splicing was observed through development. While neuroligin gene products were generally detected in the inner retina, low levels of neuroligin 1 mRNA were also detected in the photoreceptor layer. Neuroligin 3 and -4 transcripts, on the other hand, were only detected in the inner retina. At retinal synapses neuroligin 1 protein was detected in the inner plexiform layer, but its highest levels were detected in the outer plexiform layer on the tips of horizontal cell dendrites. This work lays the groundwork for future studies on the functional roles of the neuroligins within the retina.
© 2010 Wiley-Liss, Inc.
Figures








References
-
- Aartsen WM, Arsanto JP, Chauvin JP, Vos RM, Versteeg I, Cardozo BN, Bivic AL, Wijnholds J. PSD95beta regulates plasma membrane Ca2+ pump localization at the photoreceptor synapse. Mol Cell Neurosci. 2009;41:156–165. - PubMed
-
- Adler R. Curing blindness with stem cells: hope, reality, and challenges. Adv Exp Med Biol. 2008;613:3–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

