Role of interstitial branching in the development of visual corticocortical connections: a time-lapse and fixed-tissue analysis
- PMID: 21031561
- PMCID: PMC3870178
- DOI: 10.1002/cne.22502
Role of interstitial branching in the development of visual corticocortical connections: a time-lapse and fixed-tissue analysis
Abstract
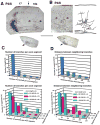
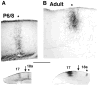
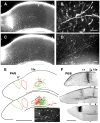
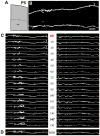
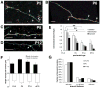
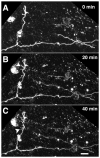
We combined fixed-tissue and time-lapse analyses to investigate the axonal branching phenomena underlying the development of topographically organized ipsilateral projections from area 17 to area 18a in the rat. These complementary approaches allowed us to relate static, large-scale information provided by traditional fixed-tissue analysis to highly dynamic, local, small-scale branching phenomena observed with two-photon time-lapse microscopy in acute slices of visual cortex. Our fixed-tissue data revealed that labeled area 17 fibers invaded area 18a gray matter at topographically restricted sites, reaching superficial layers in significant numbers by postnatal day 6 (P6). Moreover, most parental axons gave rise to only one or occasionally a small number of closely spaced interstitial branches beneath 18a. Our time-lapse data showed that many filopodium-like branches emerged along parental axons in white matter or deep layers in area 18a. Most of these filopodial branches were transient, often disappearing after several minutes to hours of exploratory extension and retraction. These dynamic behaviors decreased significantly from P4, when the projection is first forming, through the second postnatal week, suggesting that the expression of, or sensitivity to, cortical cues promoting new branch addition in the white matter is developmentally down-regulated coincident with gray matter innervation. Together, these data demonstrate that the development of topographically organized corticocortical projections in rats involves extensive exploratory branching along parental axons and invasion of cortex by only a small number of interstitial branches, rather than the widespread innervation of superficial cortical layers by an initially exuberant population of branches.
© 2010 Wiley-Liss, Inc.
Figures


References
-
- Aggoun-Zouaoui D, Innocenti GM. Juvenile visual callosal axons in kittens display origin- and fate-related morphology and distribution of arbors. Eur J Neurosci. 1994;6:1846–1863. - PubMed
-
- Barone P, Dehay C, Berland M, Kennedy H. Role of directed growth and target selection in the formation of cortical pathways: prenatal development of the projection of area V2 to area V4 in the monkey. J Comp Neurol. 1996;374:1–20. - PubMed
-
- Bradke F, Dotti CG. Differentiated neurons retain the capacity to generate axons from dendrites. Curr Biol. 2000;10:1467–1470. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

