Recombinant osteopontin in cerebral vasospasm after subarachnoid hemorrhage
- PMID: 21031580
- PMCID: PMC2967465
- DOI: 10.1002/ana.22102
Recombinant osteopontin in cerebral vasospasm after subarachnoid hemorrhage
Abstract
Objective: Osteopontin (OPN), a pleiotropic extracellular matrix glycoprotein, has been reported to be protective against ischemic lesions, but effects of OPN on vascular functions have not been investigated. The aim of this study was to assess whether recombinant OPN (r-OPN) could prevent cerebral vasospasm after subarachnoid hemorrhage (SAH) in rats.
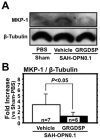
Methods: r-OPN was administered intraventricularly to rats undergoing SAH by endovascular perforation, and its protective effects were evaluated by measuring the diameter of cerebral arteries and neurobehavioral testing. Western blotting and immunofluorescence were performed to explore the underlying mechanisms. An integrin receptor antagonist GRGDSP or mitogen-activated protein kinase (MAPK) phosphatase (MKP)-1 small interfering RNA (siRNA) was also administered to r-OPN-treated SAH rats, and those effects were evaluated.
Results: Pre-SAH administration of r-OPN prevented vasospasm and neurological impairments at 24-72 hours post-SAH. r-OPN enhanced an endogenous MAPK inhibitor, MKP-1, and suppressed the phosphorylation of MAPKs, caldesmon, and heat shock protein 27 in the spastic cerebral arteries at 24 hours post-SAH. Immunofluorescence revealed that MKP-1 was induced in the arterial smooth muscle layer. GRGDSP prevented r-OPN-induced MKP-1 upregulation, and MKP-1 siRNA abolished both MAPK inactivation and anti-vasospastic effects by r-OPN. Post-SAH r-OPN treatment also prevented vasospasm.
Interpretation: r-OPN induced MKP-1 in the spastic cerebral arteries via binding to L-arginyl-glycyl-L-aspartate-dependent integrin receptors and prevented vasospasm after SAH. Therapeutic induction of MKP-1 may be a novel approach for the prevention and treatment of cerebral vasospasm.
Figures






References
-
- Suarez JI, Tarr RW, Selman WR. Aneurysmal subarachnoid hemorrhage. N Engl J Med. 2006;354:387–396. - PubMed
-
- Bederson JB, Connolly ES, Jr, Batjer HH, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40:994–1025. - PubMed
-
- Vajkoczy P, Meyer B, Weidauer S, et al. Clazosentan (AXV-034343), a selective endothelin A receptor antagonist, in the prevention of cerebral vasospasm following severe aneurysmal subarachnoid hemorrhage: results of a randomized, double-blind, placebo-controlled, multicenter phase IIa study. J Neurosurg. 2005;103:9–17. - PubMed
-
- Macdonald RL, Kassell NF, Mayer S, et al. Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke. 2008;39:3015–3021. - PubMed
-
- Meller R, Stevens SL, Minami M, et al. Neuroprotection by osteopontin in stroke. J Cereb Blood Flow Metab. 2005;25:217–225. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

