[The role of the voltage-dependent anion channels in the outer membrane of mitochondria in the regulation of cellular metabolism]
- PMID: 21033348
- PMCID: PMC4547860
[The role of the voltage-dependent anion channels in the outer membrane of mitochondria in the regulation of cellular metabolism]
Abstract
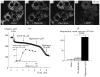
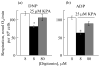
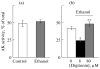
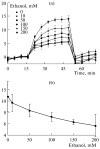
The role of voltage-dependent anion channels (VDAC/porins) of the mitochondrial outer membrane in the regulation of cell metabolism is assessed using an experimental model of ethanol toxicity in cultured hepatocytes. It is demonstrated that ethanol inhibits the phosphorylating and the uncoupled mitochondrial respiration, decreases the accessibility of mitochondrial adenylate kinase in the intermembrane space, and suppresses ureagenic respiration in the cells. Treatment with digitonin at high concentrations (>80 μM)—which creates pores in the mitochondrial outer membrane, allowing bypass of closed VDAC—restores all the processes suppressed with ethanol. It is concluded that the effect of ethanol in hepatocytes leads to global loss of mitochondrial function because of closure of VDAC, which limits the free diffusion of metabolites into the intermembrane space. Our studies also reveal the role of VDAC in the regulation of liver-specific intracellular processes such as ureagenesis. The data obtained can be used in development of pharmaceuticals that would prevent VDAC closure in mitochondria of ethanol-oxidizing liver, thus protecting liver tissue from the hepatotoxic action of alcohol.
Figures

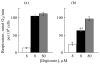
References
-
- Garcia-Rivas GJ, Torre-Amione G. Methodist DeBakey Cardiovasc J. 2009;5:2. - PubMed
-
- Kuznetsov AV, Hermann M, Saks V, Hengster P, Margreiter R. Int J Biochem Cell Biol. 2009;41:1928. - PubMed
-
- Martinelli P, Rugarli EI. Biochim Biophys Acta. 2010;1797:1. - PubMed
-
- Tylkova L. Gen Physiol Biophys. 2009;28:219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
