Biofunctionalization on alkylated silicon substrate surfaces via "click" chemistry
- PMID: 21033708
- PMCID: PMC3059218
- DOI: 10.1021/ja1025497
Biofunctionalization on alkylated silicon substrate surfaces via "click" chemistry
Abstract
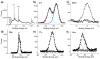
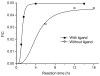
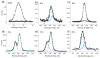
Biofunctionalization of silicon substrates is important to the development of silicon-based biosensors and devices. Compared to conventional organosiloxane films on silicon oxide intermediate layers, organic monolayers directly bound to the nonoxidized silicon substrates via Si-C bonds enhance the sensitivity of detection and the stability against hydrolytic cleavage. Such monolayers presenting a high density of terminal alkynyl groups for bioconjugation via copper-catalyzed azide-alkyne 1,3-dipolar cycloaddition (CuAAC, a "click" reaction) were reported. However, yields of the CuAAC reactions on these monolayer platforms were low. Also, the nonspecific adsorption of proteins on the resultant surfaces remained a major obstacle for many potential biological applications. Herein, we report a new type of "clickable" monolayers grown by selective, photoactivated surface hydrosilylation of α,ω-alkenynes, where the alkynyl terminal is protected with a trimethylgermanyl (TMG) group, on hydrogen-terminated silicon substrates. The TMG groups on the film are readily removed in aqueous solutions in the presence of Cu(I). Significantly, the degermanylation and the subsequent CuAAC reaction with various azides could be combined into a single step in good yields. Thus, oligo(ethylene glycol) (OEG) with an azido tag was attached to the TMG-alkyne surfaces, leading to OEG-terminated surfaces that reduced the nonspecific adsorption of protein (fibrinogen) by >98%. The CuAAC reaction could be performed in microarray format to generate arrays of mannose and biotin with varied densities on the protein-resistant OEG background. We also demonstrated that the monolayer platform could be functionalized with mannose for highly specific capturing of living targets (Escherichia coli expressing fimbriae) onto the silicon substrates.
Figures



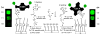
Similar articles
-
"Click" immobilization on alkylated silicon substrates: model for the study of surface bound antimicrobial peptides.Chemistry. 2011 Feb 25;17(9):2656-65. doi: 10.1002/chem.201001533. Epub 2011 Jan 24. Chemistry. 2011. PMID: 21264959 Free PMC article.
-
Rapid grafting of azido-labeled oligo(ethylene glycol)s onto an alkynyl-terminated monolayer on nonoxidized silicon via microwave-assisted "click" reaction.Langmuir. 2011 Mar 15;27(6):2437-45. doi: 10.1021/la104060j. Epub 2011 Feb 9. Langmuir. 2011. PMID: 21306165 Free PMC article.
-
Click chemistry-based functionalization on non-oxidized silicon substrates.Chem Asian J. 2011 Oct 4;6(10):2592-605. doi: 10.1002/asia.201100294. Epub 2011 Jul 12. Chem Asian J. 2011. PMID: 21751406 Review.
-
Grafting of poly(ethylene glycol) on click chemistry modified Si(100) surfaces.Langmuir. 2013 Jul 2;29(26):8355-62. doi: 10.1021/la400721c. Epub 2013 Jun 21. Langmuir. 2013. PMID: 23790067
-
Recent Advances in Recoverable Systems for the Copper-Catalyzed Azide-Alkyne Cycloaddition Reaction (CuAAC).Molecules. 2016 Sep 5;21(9):1174. doi: 10.3390/molecules21091174. Molecules. 2016. PMID: 27607998 Free PMC article. Review.
Cited by
-
Biofunctionalization of a "clickable" organic layer photochemically grafted on titanium substrates.Langmuir. 2011 Apr 19;27(8):4848-56. doi: 10.1021/la104853t. Epub 2011 Mar 21. Langmuir. 2011. PMID: 21417429 Free PMC article.
-
From the bottom up: dimensional control and characterization in molecular monolayers.Chem Soc Rev. 2013 Apr 7;42(7):2725-45. doi: 10.1039/c2cs35365b. Chem Soc Rev. 2013. PMID: 23258565 Free PMC article. Review.
-
Biofunctionalization of silicone polymers using poly(amidoamine) dendrimers and a mannose derivative for prolonged interference against pathogen colonization.Biomaterials. 2011 Jul;32(19):4336-46. doi: 10.1016/j.biomaterials.2011.02.056. Epub 2011 Mar 24. Biomaterials. 2011. PMID: 21435713 Free PMC article.
-
"Click" immobilization on alkylated silicon substrates: model for the study of surface bound antimicrobial peptides.Chemistry. 2011 Feb 25;17(9):2656-65. doi: 10.1002/chem.201001533. Epub 2011 Jan 24. Chemistry. 2011. PMID: 21264959 Free PMC article.
-
Surfaces Presenting α-Phenyl Mannoside Derivatives Enable Formation of Stable, High Coverage, Non-pathogenic Escherichia coli Biofilms against Pathogen Colonization.Biomater Sci. 2015 Jun 1;3(6):781-880. doi: 10.1039/C5BM00076A. Biomater Sci. 2015. PMID: 26029359 Free PMC article.
References
-
- Hamers RJ. Annu Rev Anal Chem. 2008;1:707–736. - PubMed
- Ciampi S, Gooding JJ. Chem-Eur J. 2010;16:5961–5968. - PubMed
- Yang WS, Butler JE, Russell JN, Hamers RJ. Analyst. 2007;132:296–306. - PubMed
- Shalek AK, Robinson JT, Karp ES, Lee JS, Ahn DR, Yoon MH, Sutton A, Jorgolli M, Gertner RS, Gujral TS, MacBeath G, Yang EG, Park H. Proc Natl Acad Sci U S A. 2010;107:1870–1875. - PMC - PubMed
- Ainslie KM, Desai TA. Lab Chip. 2008;8:1864–1878. - PMC - PubMed
- Vilan A, Yaffe O, Biller A, Salomon A, Kahn A, Cohen D. Adv Mater. 2010;22:140–159. - PubMed
- Touahir L, Moraillon A, Allongue P, Chazalviel JN, de Villeneuve CH, Ozanam F, Solomon I, Gouget-Laemmel AC. Biosens Bioelectron. 2009;25:952–955. - PubMed
-
- Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, Branner A, Chen D, Penn RD, Donoghue JP. Nature. 2006;442:164–171. - PubMed
-
- He Y, Kang ZH, Li QS, Tsang CHA, Fan CH, Lee ST. Angew Chem-Int Edit. 2009;48:128–132. - PubMed
- He Y, Su YY, Yang XB, Kang ZH, Xu TT, Zhang RQ, Fan CH, Lee ST. J Am Chem Soc. 2009;131:4434–4438. - PubMed
- Shiohara A, Hanada S, Prabakar S, Fujioka K, Lim TH, Yamamoto K, Northcote PT, Tilley RD. J Am Chem Soc. 2010;132:248–253. - PubMed
- Erogbogbo F, Yong KT, Roy I, Xu GX, Prasad PN, Swihart MT. ACS Nano. 2008;2:873–878. - PMC - PubMed
- Okamoto H, Kumai Y, Sugiyama Y, Mitsuoka T, Nakanishi K, Ohta T, Nozaki H, Yamaguchi S, Shirai S, Nakano H. J Am Chem Soc. 2010;132:2710–2718. - PubMed
- Orosco MM, Pacholski C, Miskelly GM, Sailor MJ. Adv Mater. 2006;18:1393–1396.
- Tilley RD, Yamamoto K. Adv Mater. 2006;18:2053–2056.
-
- Rosso-Vasic M, Spruijt E, Popovic Z, Overgaag K, van Lagen B, Grandidier B, Vanmaekelbergh D, Dominguez-Gutierrez D, De Cola L, Zuilhof H. J Mater Chem. 2009;19:5926–5933.
-
- Gao XPA, Zheng GF, Lieber CM. Nano Lett. 2010;10:547–552. - PMC - PubMed
- Ben Ishai M, Patolsky F. J Am Chem Soc. 2009;131:3679–3689. - PubMed
- Bunimovich YL, Shin YS, Yeo WS, Amori M, Kwong G, Heath JR. J Am Chem Soc. 2006;128:16323–16331. - PMC - PubMed
- Cohen-Karni T, Timko BP, Weiss LE, Lieber CM. Proc Natl Acad Sci U S A. 2009;106:7309–7313. - PMC - PubMed
- Martinez JA, Misra N, Wang YM, Stroeve P, Grigoropoulos CP, Noy A. Nano Lett. 2009;9:1121–1126. - PubMed
- Patolsky F, Zheng G, Lieber CM. Nanomedicine. 2006;1:51–65. - PubMed
- Wang WU, Chen C, Lin KH, Fang Y, Lieber CM. Proc Natl Acad Sci U S A. 2005;102:3208–3212. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

