Systemic treatment with liver X receptor agonists raises apolipoprotein E, cholesterol, and amyloid-β peptides in the cerebral spinal fluid of rats
- PMID: 21034469
- PMCID: PMC2988784
- DOI: 10.1186/1750-1326-5-44
Systemic treatment with liver X receptor agonists raises apolipoprotein E, cholesterol, and amyloid-β peptides in the cerebral spinal fluid of rats
Abstract
Background: Apolipoprotein E (apoE) is a major cholesterol transport protein found in association with brain amyloid from Alzheimer's disease (AD) patients and the ε4 allele of apoE is a genetic risk factor for AD. Previous studies have shown that apoE forms a stable complex with amyloid β (Aβ) peptides in vitro and that the state of apoE lipidation influences the fate of brain Aβ, i.e., lipid poor apoE promotes Aβ aggregation/deposition while fully lipidated apoE favors Aβ degradation/clearance. In the brain, apoE levels and apoE lipidation are regulated by the liver X receptors (LXRs).
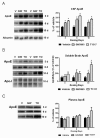
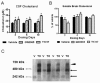
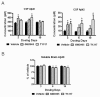
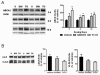
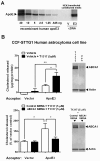
Results: We investigated the hypothesis that increased apoE levels and lipidation induced by LXR agonists facilitates Aβ efflux from the brain to the cerebral spinal fluid (CSF). We also examined if the brain expression of major apoE receptors potentially involved in apoE-mediated Aβ clearance was altered by LXR agonists. ApoE, cholesterol, Aβ40, and Aβ42 levels were all significantly elevated in the CSF of rats after only 3 days of treatment with LXR agonists. A significant reduction in soluble brain Aβ40 levels was also detected after 6 days of LXR agonist treatment.
Conclusions: Our novel findings suggest that central Aβ lowering caused by LXR agonists appears to involve an apoE/cholesterol-mediated transport of Aβ to the CSF and that differences between the apoE isoforms in mediating this clearance pathway may explain why individuals carrying one or two copies of APOE ε4 have increased risk for AD.
Figures

Similar articles
-
Greasing the wheels of Abeta clearance in Alzheimer's disease: the role of lipids and apolipoprotein E.Biofactors. 2009 May-Jun;35(3):239-48. doi: 10.1002/biof.37. Biofactors. 2009. PMID: 19472365 Review.
-
Effect of LXR/RXR agonism on brain and CSF Aβ40 levels in rats.F1000Res. 2016 Feb 4;5:138. doi: 10.12688/f1000research.7868.2. eCollection 2016. F1000Res. 2016. PMID: 27239272 Free PMC article.
-
Combined Liver X Receptor/Peroxisome Proliferator-activated Receptor γ Agonist Treatment Reduces Amyloid β Levels and Improves Behavior in Amyloid Precursor Protein/Presenilin 1 Mice.J Biol Chem. 2015 Aug 28;290(35):21591-602. doi: 10.1074/jbc.M115.652008. Epub 2015 Jul 10. J Biol Chem. 2015. PMID: 26163517 Free PMC article.
-
Liver X receptor agonist treatment significantly affects phenotype and transcriptome of APOE3 and APOE4 Abca1 haplo-deficient mice.PLoS One. 2017 Feb 27;12(2):e0172161. doi: 10.1371/journal.pone.0172161. eCollection 2017. PLoS One. 2017. PMID: 28241068 Free PMC article.
-
Liver X receptor-mediated gene regulation and cholesterol homeostasis in brain: relevance to Alzheimer's disease therapeutics.Curr Alzheimer Res. 2007 Apr;4(2):179-84. doi: 10.2174/156720507780362173. Curr Alzheimer Res. 2007. PMID: 17430244 Review.
Cited by
-
Liver X Receptor Activation with an Intranasal Polymer Therapeutic Prevents Cognitive Decline without Altering Lipid Levels.ACS Nano. 2021 Mar 23;15(3):4678-4687. doi: 10.1021/acsnano.0c09159. Epub 2021 Mar 5. ACS Nano. 2021. PMID: 33666411 Free PMC article.
-
Total protein is an effective loading control for cerebrospinal fluid western blots.J Neurosci Methods. 2015 Aug 15;251:72-82. doi: 10.1016/j.jneumeth.2015.05.011. Epub 2015 May 22. J Neurosci Methods. 2015. PMID: 26004848 Free PMC article.
-
Glymphatic distribution of CSF-derived apoE into brain is isoform specific and suppressed during sleep deprivation.Mol Neurodegener. 2016 Dec 8;11(1):74. doi: 10.1186/s13024-016-0138-8. Mol Neurodegener. 2016. PMID: 27931262 Free PMC article.
-
Ligands of Therapeutic Utility for the Liver X Receptors.Molecules. 2017 Jan 5;22(1):88. doi: 10.3390/molecules22010088. Molecules. 2017. PMID: 28067791 Free PMC article. Review.
-
A randomized controlled study to evaluate the effect of bexarotene on amyloid-β and apolipoprotein E metabolism in healthy subjects.Alzheimers Dement (N Y). 2016 Jun 17;2(2):110-120. doi: 10.1016/j.trci.2016.06.001. eCollection 2016 Jun. Alzheimers Dement (N Y). 2016. PMID: 29067298 Free PMC article.
References
-
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

