Manufacture of IRDye800CW-coupled Fe3O4 nanoparticles and their applications in cell labeling and in vivo imaging
- PMID: 21034487
- PMCID: PMC2984479
- DOI: 10.1186/1477-3155-8-25
Manufacture of IRDye800CW-coupled Fe3O4 nanoparticles and their applications in cell labeling and in vivo imaging
Abstract
Background: In recent years, near-infrared fluorescence (NIRF)-labeled iron nanoparticles have been synthesized and applied in a number of applications, including the labeling of human cells for monitoring the engraftment process, imaging tumors, sensoring the in vivo molecular environment surrounding nanoparticles and tracing their in vivo biodistribution. These studies demonstrate that NIRF-labeled iron nanoparticles provide an efficient probe for cell labeling. Furthermore, the in vivo imaging studies show excellent performance of the NIR fluorophores. However, there is a limited selection of NIRF-labeled iron nanoparticles with an optimal wavelength for imaging around 800 nm, where tissue autofluorescence is minimal. Therefore, it is necessary to develop additional alternative NIRF-labeled iron nanoparticles for application in this area.
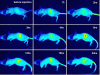
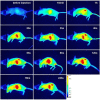
Results: This study manufactured 12-nm DMSA-coated Fe3O4 nanoparticles labeled with a near-infrared fluorophore, IRDye800CW (excitation/emission, 774/789 nm), to investigate their applicability in cell labeling and in vivo imaging. The mouse macrophage RAW264.7 was labeled with IRDye800CW-labeled Fe3O4 nanoparticles at concentrations of 20, 30, 40, 50, 60, 80 and 100 μg/ml for 24 h. The results revealed that the cells were efficiently labeled by the nanoparticles, without any significant effect on cell viability. The nanoparticles were injected into the mouse via the tail vein, at dosages of 2 or 5 mg/kg body weight, and the mouse was discontinuously imaged for 24 h. The results demonstrated that the nanoparticles gradually accumulated in liver and kidney regions following injection, reaching maximum concentrations at 6 h post-injection, following which they were gradually removed from these regions. After tracing the nanoparticles throughout the body it was revealed that they mainly distributed in three organs, the liver, spleen and kidney. Real-time live-body imaging effectively reported the dynamic process of the biodistribution and clearance of the nanoparticles in vivo.
Conclusion: IRDye800CW-labeled Fe3O4 nanoparticles provide an effective probe for cell-labeling and in vivo imaging.
Figures






References
-
- Tiefenauer L. In: Nanotechnology in Biology and Medicine. Tuan Vo-Dinh, editor. Boca Raton: CRC Press; 2007. Magnetic Nanoparticles as Contrast Agents for Medical Diagnosis. 29/10.
LinkOut - more resources
Full Text Sources
Miscellaneous

