Fast growth associated with aberrant vasculature and hypoxia in fibroblast growth factor 8b (FGF8b) over-expressing PC-3 prostate tumour xenografts
- PMID: 21034500
- PMCID: PMC2984431
- DOI: 10.1186/1471-2407-10-596
Fast growth associated with aberrant vasculature and hypoxia in fibroblast growth factor 8b (FGF8b) over-expressing PC-3 prostate tumour xenografts
Abstract
Background: Prostate tumours are commonly poorly oxygenated which is associated with tumour progression and development of resistance to chemotherapeutic drugs and radiotherapy. Fibroblast growth factor 8b (FGF8b) is a mitogenic and angiogenic factor, which is expressed at an increased level in human prostate tumours and is associated with a poor prognosis. We studied the effect of FGF8b on tumour oxygenation and growth parameters in xenografts in comparison with vascular endothelial growth factor (VEGF)-expressing xenografts, representing another fast growing and angiogenic tumour model.
Methods: Subcutaneous tumours of PC-3 cells transfected with FGF8b, VEGF or empty (mock) vectors were produced and studied for vascularity, cell proliferation, glucose metabolism and oxygenation. Tumours were evaluated by immunohistochemistry (IHC), flow cytometry, use of radiolabelled markers of energy metabolism ([18F]FDG) and hypoxia ([18F]EF5), and intratumoral polarographic measurements of pO2.
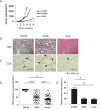
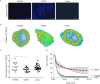
Results: Both FGF8b and VEGF tumours grew rapidly in nude mice and showed highly vascularised morphology. Perfusion studies, pO2 measurements, [18F]EF5 and [18F]FDG uptake as well as IHC staining for glucose transport protein (GLUT1) and hypoxia inducible factor (HIF) 1 showed that VEGF xenografts were well-perfused and oxygenised, as expected, whereas FGF8b tumours were as hypoxic as mock tumours. These results suggest that FGF8b-induced tumour capillaries are defective. Nevertheless, the growth rate of hypoxic FGF8b tumours was highly increased, as that of well-oxygenised VEGF tumours, when compared with hypoxic mock tumour controls.
Conclusion: FGF8b is able to induce fast growth in strongly hypoxic tumour microenvironment whereas VEGF-stimulated growth advantage is associated with improved perfusion and oxygenation of prostate tumour xenografts.
Figures




Similar articles
-
A neutralizing anti-fibroblast growth factor (FGF) 8 monoclonal antibody shows anti-tumor activity against FGF8b-expressing LNCaP xenografts in androgen-dependent and -independent conditions.Prostate. 2008 May 1;68(6):640-50. doi: 10.1002/pros.20728. Prostate. 2008. PMID: 18213631
-
Angiogenesis, hypoxia and VEGF expression during tumour growth in a human xenograft tumour model.Microvasc Res. 2009 Mar;77(2):96-103. doi: 10.1016/j.mvr.2008.11.002. Epub 2008 Dec 7. Microvasc Res. 2009. PMID: 19118564
-
Hypoxia-induced angiogenesis and vascular endothelial growth factor secretion in human melanoma.Br J Cancer. 1998 Mar;77(6):897-902. doi: 10.1038/bjc.1998.148. Br J Cancer. 1998. PMID: 9528831 Free PMC article.
-
The relevance of a hypoxic tumour microenvironment in prostate cancer.BJU Int. 2010 Jan;105(1):8-13. doi: 10.1111/j.1464-410X.2009.08921.x. Epub 2009 Nov 4. BJU Int. 2010. PMID: 19889065 Review.
-
Targeting hypoxia in solid and haematological malignancies.J Exp Clin Cancer Res. 2022 Nov 2;41(1):318. doi: 10.1186/s13046-022-02522-y. J Exp Clin Cancer Res. 2022. PMID: 36320041 Free PMC article. Review.
Cited by
-
A detailed protocol for a rapid analysis of testicular cell populations using flow cytometry.Andrology. 2015 Sep;3(5):947-55. doi: 10.1111/andr.12066. Epub 2015 Aug 7. Andrology. 2015. PMID: 26256546 Free PMC article.
-
FGF8 promotes colorectal cancer growth and metastasis by activating YAP1.Oncotarget. 2015 Jan 20;6(2):935-52. doi: 10.18632/oncotarget.2822. Oncotarget. 2015. PMID: 25473897 Free PMC article.
-
Hypoxia-activated prodrug TH-302 decreased survival rate of canine lymphoma cells under hypoxic condition.PLoS One. 2017 May 10;12(5):e0177305. doi: 10.1371/journal.pone.0177305. eCollection 2017. PLoS One. 2017. PMID: 28489881 Free PMC article.
-
Critical evaluation of the subcutaneous engraftments of hormone naïve primary prostate cancer.Transl Androl Urol. 2020 Jun;9(3):1120-1134. doi: 10.21037/tau.2020.03.38. Transl Androl Urol. 2020. PMID: 32676396 Free PMC article.
-
Hypoxia regulates Toll-like receptor-9 expression and invasive function in human brain cancer cells in vitro.Oncol Lett. 2014 Jul;8(1):266-274. doi: 10.3892/ol.2014.2095. Epub 2014 Apr 25. Oncol Lett. 2014. PMID: 24959259 Free PMC article.
References
-
- Jokilehto T, Rantanen K, Luukkaa M, Heikkinen P, Grenman R, Minn H, Kronqvist P, Jaakkola PM. Overexpression and nuclear translocation of hypoxia-inducible factor prolyl hydroxylase PHD2 in head and neck squamous cell carcinoma is associated with tumor aggressiveness. Clin Cancer Res. 2006;12(4):1080–7. doi: 10.1158/1078-0432.CCR-05-2022. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

