Pro-inflammatory cytokines derived from West Nile virus (WNV)-infected SK-N-SH cells mediate neuroinflammatory markers and neuronal death
- PMID: 21034511
- PMCID: PMC2984415
- DOI: 10.1186/1742-2094-7-73
Pro-inflammatory cytokines derived from West Nile virus (WNV)-infected SK-N-SH cells mediate neuroinflammatory markers and neuronal death
Abstract
Background: WNV-associated encephalitis (WNVE) is characterized by increased production of pro-inflammatory mediators, glial cells activation and eventual loss of neurons. WNV infection of neurons is rapidly progressive and destructive whereas infection of non-neuronal brain cells is limited. However, the role of neurons and pathological consequences of pro-inflammatory cytokines released as a result of WNV infection is unclear. Therefore, the objective of this study was to examine the role of key cytokines secreted by WNV-infected neurons in mediating neuroinflammatory markers and neuronal death.
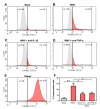
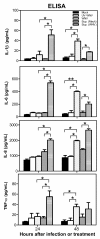
Methods: A transformed human neuroblastoma cell line, SK-N-SH, was infected with WNV at multiplicity of infection (MOI)-1 and -5, and WNV replication kinetics and expression profile of key pro-inflammatory cytokines were analyzed by plaque assay, qRT-PCR, and ELISA. Cell death was measured in SK-N-SH cell line in the presence and absence of neutralizing antibodies against key pro-inflammatory cytokines using cell viability assay, TUNEL and flow cytometry. Further, naïve primary astrocytes were treated with UV-inactivated supernatant from mock- and WNV-infected SK-N-SH cell line and the activation of astrocytes was measured using flow cytometry and ELISA.
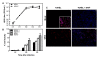
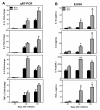
Results: WNV-infected SK-N-SH cells induced the expression of IL-1β, -6, -8, and TNF-α in a dose- and time-dependent manner, which coincided with increase in virus-induced cell death. Treatment of cells with anti-IL-1β or -TNF-α resulted in significant reduction of the neurotoxic effects of WNV. Furthermore treatment of naïve astrocytes with UV-inactivated supernatant from WNV-infected SK-N-SH cell line increased expression of glial fibrillary acidic protein and key inflammatory cytokines.
Conclusion: Our results for the first time suggest that neurons are one of the potential sources of pro-inflammatory cytokines in WNV-infected brain and these neuron-derived cytokines contribute to WNV-induced neurotoxicity. Moreover, cytokines released from neurons also mediate the activation of astrocytes. Our data define specific role(s) of WNV-induced pro-inflammatory cytokines and provide a framework for the development of anti-inflammatory drugs as much-needed therapeutic interventions to limit symptoms associated with WNVE.
Figures


Similar articles
-
Intrinsic Innate Immune Responses Control Viral Growth and Protect against Neuronal Death in an Ex Vivo Model of West Nile Virus-Induced Central Nervous System Disease.J Virol. 2021 Aug 25;95(18):e0083521. doi: 10.1128/JVI.00835-21. Epub 2021 Aug 25. J Virol. 2021. PMID: 34190599 Free PMC article.
-
Reduced immune cell infiltration and increased pro-inflammatory mediators in the brain of Type 2 diabetic mouse model infected with West Nile virus.J Neuroinflammation. 2014 Apr 21;11:80. doi: 10.1186/1742-2094-11-80. J Neuroinflammation. 2014. PMID: 24750819 Free PMC article.
-
Changes in cytokine and chemokine profiles in mouse serum and brain, and in human neural cells, upon tick-borne encephalitis virus infection.J Neuroinflammation. 2019 Nov 7;16(1):205. doi: 10.1186/s12974-019-1596-z. J Neuroinflammation. 2019. PMID: 31699097 Free PMC article.
-
Immunology of West Nile Virus Infection and the Role of Alpha-Synuclein as a Viral Restriction Factor.Viral Immunol. 2019 Jan/Feb;32(1):38-47. doi: 10.1089/vim.2018.0075. Epub 2018 Sep 15. Viral Immunol. 2019. PMID: 30222521 Review.
-
The Role of Microglia during West Nile Virus Infection of the Central Nervous System.Vaccines (Basel). 2020 Aug 28;8(3):485. doi: 10.3390/vaccines8030485. Vaccines (Basel). 2020. PMID: 32872152 Free PMC article. Review.
Cited by
-
Neuromuscular manifestations of west nile virus infection.Front Neurol. 2012 Mar 21;3:37. doi: 10.3389/fneur.2012.00037. eCollection 2012. Front Neurol. 2012. PMID: 22461779 Free PMC article.
-
Z-DNA-Binding Protein 1 Is Critical for Controlling Virus Replication and Survival in West Nile Virus Encephalitis.Front Microbiol. 2019 Sep 11;10:2089. doi: 10.3389/fmicb.2019.02089. eCollection 2019. Front Microbiol. 2019. PMID: 31572318 Free PMC article.
-
Viral Encephalitis and Neurologic Diseases: Focus on Astrocytes.Trends Mol Med. 2018 Nov;24(11):950-962. doi: 10.1016/j.molmed.2018.09.001. Epub 2018 Oct 9. Trends Mol Med. 2018. PMID: 30314877 Free PMC article. Review.
-
Lack of Interferon (IFN) Regulatory Factor 8 Associated with Restricted IFN-γ Response Augmented Japanese Encephalitis Virus Replication in the Mouse Brain.J Virol. 2021 Oct 13;95(21):e0040621. doi: 10.1128/JVI.00406-21. Epub 2021 Aug 11. J Virol. 2021. PMID: 34379515 Free PMC article.
-
Usutu virus and West Nile virus use a transcellular route of neuroinvasion across an in vitro model of the human blood-brain barrier.Npj Viruses. 2024 Jul 25;2(1):32. doi: 10.1038/s44298-024-00034-4. Npj Viruses. 2024. PMID: 40295794 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

