Comparison of modification sites formed on human serum albumin at various stages of glycation
- PMID: 21034726
- PMCID: PMC3053033
- DOI: 10.1016/j.cca.2010.10.018
Comparison of modification sites formed on human serum albumin at various stages of glycation
Abstract
Background: Many of the complications encountered during diabetes can be linked to the non-enzymatic glycation of proteins, including human serum albumin (HSA). However, there is little information regarding how the glycation pattern of HSA changes as the total extent of glycation is varied. The goal of this study was to identify and conduct a semi-quantitative comparison of the glycation products on HSA that are produced in the presence of various levels of glycation.
Methods: Three glycated HSA samples were prepared in vitro by incubating physiological concentrations of HSA with 15 mmol/l glucose for 2 or 5 weeks, or with 30 mmol/l glucose for 4 weeks. These samples were then digested and examined by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) to identify the glycation products that were formed.
Results: It was found that the glycation pattern of HSA changed with its overall extent of total glycation. Many modifications including previously-reported primary glycation sites (e.g., K199, K281, and the N-terminus) were consistently found in the tested samples. Lysines 199 and 281, as well as arginine 428, contained the most consistently identified and abundant glycation products. Lysines 93, 276, 286, 414, 439, and 524/525, as well as the N-terminus and arginines 98, 197, and 521, were also found to be modified at various degrees of HSA glycation.
Conclusions: The glycation pattern of HSA was found to vary with different levels of total glycation and included modifications at the 2 major drug binding sites on this protein. This result suggests that different modified forms of HSA, both in terms of the total extent of glycation and glycation pattern, may be found at various stages of diabetes. The clinical implication of these results is that the binding of HSA to some drug may be altered at various stages of diabetes as the extent of glycation and types of modifications in this protein are varied.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures

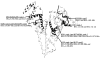
Similar articles
-
Quantitative analysis of glycation sites on human serum albumin using (16)O/(18)O-labeling and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry.Clin Chim Acta. 2010 Aug 5;411(15-16):1102-10. doi: 10.1016/j.cca.2010.04.007. Epub 2010 Apr 13. Clin Chim Acta. 2010. PMID: 20394739 Free PMC article.
-
Quantitative analysis of glycation patterns in human serum albumin using 16O/18O-labeling and MALDI-TOF MS.Clin Chim Acta. 2011 Aug 17;412(17-18):1606-15. doi: 10.1016/j.cca.2011.05.012. Epub 2011 May 13. Clin Chim Acta. 2011. PMID: 21601565 Free PMC article.
-
Characterization of glycation adducts on human serum albumin by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry.Clin Chim Acta. 2007 Oct;385(1-2):48-60. doi: 10.1016/j.cca.2007.06.011. Epub 2007 Jun 23. Clin Chim Acta. 2007. PMID: 17707360 Free PMC article.
-
Review: Glycation of human serum albumin.Clin Chim Acta. 2013 Oct 21;425:64-76. doi: 10.1016/j.cca.2013.07.013. Epub 2013 Jul 24. Clin Chim Acta. 2013. PMID: 23891854 Free PMC article. Review.
-
Analysis of protein glycation products by matrix-assisted laser desorption ionization time-of-flight mass spectrometry.Curr Med Chem. 2004 Aug;11(16):2185-93. doi: 10.2174/0929867043364649. Curr Med Chem. 2004. PMID: 15279557 Review.
Cited by
-
Characterization of tolazamide binding with glycated and normal human serum albumin by using high-performance affinity chromatography.J Pharm Biomed Anal. 2019 Mar 20;166:273-280. doi: 10.1016/j.jpba.2019.01.025. Epub 2019 Jan 14. J Pharm Biomed Anal. 2019. PMID: 30682693 Free PMC article.
-
Site-specific Labeling of a Protein Lysine Residue By Novel Kinetic Labeling Combinatorial Libraries.Comput Struct Biotechnol J. 2014 Mar 26;9:e201403001. doi: 10.5936/csbj.201403001. eCollection 2014. Comput Struct Biotechnol J. 2014. PMID: 24757504 Free PMC article.
-
Multiple Glycation Sites in Blood Plasma Proteins as an Integrated Biomarker of Type 2 Diabetes Mellitus.Int J Mol Sci. 2019 May 10;20(9):2329. doi: 10.3390/ijms20092329. Int J Mol Sci. 2019. PMID: 31083443 Free PMC article.
-
Aqueous extract of some indigenous medicinal plants inhibits glycation at multiple stages and protects erythrocytes from oxidative damage-an in vitro study.J Food Sci Technol. 2015 Apr;52(4):1911-23. doi: 10.1007/s13197-013-1211-8. Epub 2013 Nov 26. J Food Sci Technol. 2015. PMID: 25829572 Free PMC article.
-
Characterization of binding by repaglinide and nateglinide with glycated human serum albumin using high-performance affinity microcolumns.J Sep Sci. 2022 Dec;45(23):4176-4186. doi: 10.1002/jssc.202200686. Epub 2022 Oct 3. J Sep Sci. 2022. PMID: 36168862 Free PMC article.
References
-
- Li W, Ota K, Nakamura J, Naruse K, Nakashima E, Oiso Y, et al. Antiglycation effect of glyclazide on in vitro AGE formation from glucose and methylglyoxal. Exp Biol Med. 2008;233:176–9. - PubMed
-
- Peters T. All about Albumin: Biochemistry, Genetics, and Medical Applications. San Diego: Academic Press; 1996.
-
- Makita Z, Vlassara H, Cerami A, Bucala R. Immunochemical detection of advanced glycosylation end products in vivo. J Biol Chem. 1992;267:5133–8. - PubMed
-
- Syrovy I. Glycation of albumin: Reaction with glucose, fructose, galactose, ribose or glyceraldehyde measured using four methods. J Biochem Biophys Meth. 1994;28:115–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

