Apotransferrin protects cortical neurons from hemoglobin toxicity
- PMID: 21034753
- PMCID: PMC3039873
- DOI: 10.1016/j.neuropharm.2010.10.015
Apotransferrin protects cortical neurons from hemoglobin toxicity
Abstract
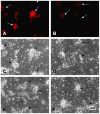
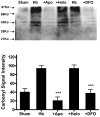
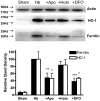
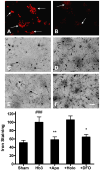
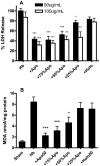
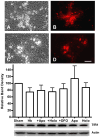
The protective effect of iron chelators in experimental models of intracerebral hemorrhage suggests that nonheme iron may contribute to injury to perihematomal cells. Therapy with high affinity iron chelators is limited by their toxicity, which may be due in part to sequestration of metals in an inaccessible complex. Transferrin is unique in chelating iron with very high affinity while delivering it to cells as needed via receptor-mediated endocytosis. However, its efficacy against iron-mediated neuronal injury has never been described, and was therefore evaluated in this study using an established cell culture model of hemoglobin neurotoxicity. At concentrations similar to that of CSF transferrin (50-100 micrograms/ml), both iron-saturated holotransferrin and apotransferrin were nontoxic per se. Overnight exposure to 3 μM purified human hemoglobin in serum-free culture medium resulted in death, as measured by lactate dehydrogenase release assay, of about three-quarters of neurons. Significant increases in culture iron, malondialdehyde, protein carbonyls, ferritin and heme oxygenase-1 were also observed. Holotransferrin had no effect on these parameters, but all were attenuated by 50-100 micrograms/ml apotransferrin. The effect of apotransferrin was very similar to that of deferoxamine at a concentration that provided equivalent iron binding capacity, and was not antagonized by concomitant treatment with holotransferrin. Transferrin receptor-1 expression was localized to neurons and was not altered by hemoglobin or transferrin treatment. These results suggest that apotransferrin may mitigate the neurotoxicity of hemoglobin after intracerebral hemorrhage. Increasing its concentration in perihematomal tissue may be beneficial.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures

References
-
- Balla G, Jacob HS, Balla J, Rosenberg M, Nath K, Apple F, Eaton JW, Vercellotti GM. Ferritin: A cytoprotective strategem of endothelium. J Biol Chem. 1992;267:18148–18153. - PubMed
-
- Benvenisti-Zarom L, Chen-Roetling J, Regan RF. Inhibition of the ERK/MAP kinase pathway attenuates heme oxygenase-1 expression and heme-mediated neuronal injury. Neurosci Lett. 2006;398:230–234. - PubMed
-
- Burdo JR, Antonetti DA, Wolpert EB, Connor JR. Mechanisms and regulation of transferrin and iron transport in a model blood-brain barrier system. Neuroscience. 2003;121:883–890. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

