Cross-genotypic polyclonal anti-HCV antibodies from human ascitic fluid
- PMID: 21034775
- PMCID: PMC3018694
- DOI: 10.1016/j.jviromet.2010.10.020
Cross-genotypic polyclonal anti-HCV antibodies from human ascitic fluid
Abstract
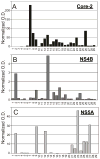
Many anti-HCV antibodies are available, but more are needed for research and clinical applications. This study examines whether ascitic fluid from cirrhotic patients could be a source of reagent-grade antibodies. Ascitic fluid from 29 HCV patients was screened by ELISA for anti-HCV antibodies against three viral proteins: core, NS4B, and NS5A. Significant patient-to-patient variability in anti-HCV antibody titers was observed. Total ascitic fluid IgG purified by Protein-A chromatography reacted with HCV proteins in immunoblots, cell extracts, and replicon-expressing cells. Affinity-purification using synthetic peptides as bait allowed the preparation of cross-genotypic antibodies directed against pre-selected regions of HCV core, NS4B, and NS5A proteins. The performance of the polyclonal antibodies was comparable to that of monoclonal antibodies. Anti-NS4B antibody preparations reacted with genotype 1a, 1b, and 2a NS4B proteins in immunoblots and allowed NS4B to be localized in replicon-expressing cells. Ascitic fluid is an abundant source of human polyclonal cross-genotypic antibodies that can be used as an alternative to blood. This study shows the utility of selectively purifying human polyclonal antibodies from ascitic fluid. Affinity purification allows antibodies to be selected that are comparable to monoclonal antibodies in their ability to react with targeted regions of viral proteins.
Copyright © 2010 Elsevier B.V. All rights reserved.
Conflict of interest statement
There were no conflicts of interests from the authors of this article.
Figures




Similar articles
-
Antigenic variation of core, NS3, and NS5 proteins among genotypes of hepatitis C virus.J Clin Microbiol. 1997 Dec;35(12):3062-70. doi: 10.1128/jcm.35.12.3062-3070.1997. J Clin Microbiol. 1997. PMID: 9399495 Free PMC article.
-
[Quantitative assay of anti-HCV core antibody with JCC-2 ELISA: anti-HCV core IgG antibody].Nihon Rinsho. 1995 Sep;53 Suppl(Pt 1):247-52. Nihon Rinsho. 1995. PMID: 7563711 Review. Japanese. No abstract available.
-
Immunogenicity of variable regions of hepatitis C virus proteins: selection and modification of peptide epitopes to assess hepatitis C virus genotypes by ELISA.J Gen Virol. 1999 Mar;80 ( Pt 3):727-738. doi: 10.1099/0022-1317-80-3-727. J Gen Virol. 1999. PMID: 10092013
-
Hepatitis C virus core, NS3, NS4B and NS5A are the major immunogenic proteins in humoral immunity in chronic HCV infection.Virol J. 2009 Jun 23;6:84. doi: 10.1186/1743-422X-6-84. Virol J. 2009. PMID: 19549310 Free PMC article.
-
[Antibodies generated against hepatitis C virus (HCV) core antigen: IgM and IgA antibody against HCV core].Nihon Rinsho. 1995 Sep;53 Suppl(Pt 1):253-9. Nihon Rinsho. 1995. PMID: 7563714 Review. Japanese. No abstract available.
Cited by
-
Characterization of differential antibody production against hepatitis C virus in different HCV infection status.Virol J. 2016 Jun 30;13:116. doi: 10.1186/s12985-016-0572-9. Virol J. 2016. PMID: 27357382 Free PMC article.
-
Relationship between humoral response against hepatitis C virus and disease overcome.Springerplus. 2014 Jan 27;3:56. doi: 10.1186/2193-1801-3-56. eCollection 2014. Springerplus. 2014. PMID: 24516785 Free PMC article.
References
-
- Knodell RG, Conrad ME, Ginsberg AL, Bell CJ. Efficacy of prophylactic gamma-globulin in preventing non-A, non-B post-transfusion hepatitis. Lancet. 1976;1:557–561. - PubMed
-
- Sanchez-Quijano A, Pineda JA, Lissen E, Leal M, Diaz-Torres MA, Garcia De Pesquera F, Rivera F, Castro R, Munoz J. Prevention of post-transfusion non-A, non-B hepatitis by non-specific immunoglobulin in heart surgery patients. Lancet. 1988;1:1245–1249. - PubMed
-
- Piazza M, Sagliocca L, Tosone G, Guadagnino V, Stazi MA, Orlando R, Borgia G, Rosa D, Abrignani S, Palumbo F, Manzin A, Clementi M. Sexual transmission of hepatitis C virus and prevention with intramuscular immunoglobulin. AIDS Patient Care STDS. 1998;12:611–618. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 5T32AI007623-08/AI/NIAID NIH HHS/United States
- R01 DA031095/DA/NIDA NIH HHS/United States
- GM064118/GM/NIGMS NIH HHS/United States
- R01 DK066939/DK/NIDDK NIH HHS/United States
- DK066939/DK/NIDDK NIH HHS/United States
- T32 AI007623/AI/NIAID NIH HHS/United States
- R25 GM064118/GM/NIGMS NIH HHS/United States
- R01 DK090317/DK/NIDDK NIH HHS/United States
- F31AI081530/AI/NIAID NIH HHS/United States
- R01 DA016156/DA/NIDA NIH HHS/United States
- R01 CA057973/CA/NCI NIH HHS/United States
- CA57973/CA/NCI NIH HHS/United States
- N01 AI040034/AI/NIAID NIH HHS/United States
- T32 DK007202/DK/NIDDK NIH HHS/United States
- DK090317/DK/NIDDK NIH HHS/United States
- DA031095/DA/NIDA NIH HHS/United States
- DA016156/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

