Innate signaling networks in mucosal IgA class switching
- PMID: 21034970
- PMCID: PMC3046556
- DOI: 10.1016/B978-0-12-381300-8.00002-2
Innate signaling networks in mucosal IgA class switching
Abstract
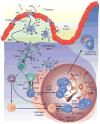
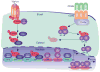
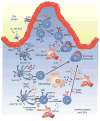
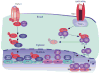
The past 20 years have seen a growing interest over the control of adaptive immune responses by the innate immune system. In particular, considerable attention has been paid to the mechanisms by which antigen-primed dendritic cells orchestrate the differentiation of T cells. Additional studies have elucidated the pathways followed by T cells to initiate immunoglobulin responses in B cells. In this review, we discuss recent advances on the mechanisms by which intestinal bacteria, epithelial cells, dendritic cells, and macrophages cross talk with intestinal T cells and B cells to induce frontline immunoglobulin A class switching and production.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
References
-
- Abreu MT. Toll-like receptor signalling in the intestinal epithelium: How bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10:131–144. - PubMed
-
- Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8:411–420. - PubMed
-
- Avery DT, Deenick EK, Ma CS, Suryani S, Simpson N, Chew GY, Chan TD, Palendira U, Bustamante J, Boisson-Dupuis S, Choo S, Bleasel KE, et al. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. J Exp Med. 2010;207:155–171. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

