Fibrochondrogenesis results from mutations in the COL11A1 type XI collagen gene
- PMID: 21035103
- PMCID: PMC2978944
- DOI: 10.1016/j.ajhg.2010.10.009
Fibrochondrogenesis results from mutations in the COL11A1 type XI collagen gene
Abstract
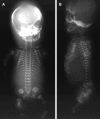
Fibrochondrogenesis is a severe, autosomal-recessive, short-limbed skeletal dysplasia. In a single case of fibrochondrogenesis, whole-genome SNP genotyping identified unknown ancestral consanguinity by detecting three autozygous regions. Because of the predominantly skeletal nature of the phenotype, the 389 genes localized to the autozygous intervals were prioritized for mutation analysis by correlation of their expression with known cartilage-selective genes via the UCLA Gene Expression Tool, UGET. The gene encoding the α1 chain of type XI collagen (COL11A1) was the only cartilage-selective gene among the three candidate intervals. Sequence analysis of COL11A1 in two genetically independent fibrochondrogenesis cases demonstrated that each was a compound heterozygote for a loss-of-function mutation on one allele and a mutation predicting substitution for a conserved triple-helical glycine residue on the other. The parents who were carriers of missense mutations had myopia. Early-onset hearing loss was noted in both parents who carried a loss-of-function allele, suggesting COL11A1 as a locus for mild, dominantly inherited hearing loss. These findings identify COL11A1 as a locus for fibrochondrogenesis and indicate that there might be phenotypic manifestations among carriers.
Copyright © 2010 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures

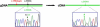
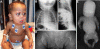
References
-
- Lazzaroni-Fossati F., Stanescu V., Stanescu R., Serra G., Magliano P., Maroteaux P. Fibrochondrogenesis. Arch. Fr. Pediatr. 1978;35:1096–1104. - PubMed
-
- Whitley C.B., Langer L.O., Jr., Ophoven J., Gilbert E.F., Gonzalez C.H., Mammel M., Coleman M., Rosemberg S., Rodriques C.J., Sibley R. Fibrochondrogenesis: Lethal, autosomal recessive chondrodysplasia with distinctive cartilage histopathology. Am. J. Med. Genet. 1984;19:265–275. - PubMed
-
- Eteson D.J., Adomian G.E., Ornoy A., Koide T., Sugiura Y., Calabro A., Lungarotti S., Mastroiacovo P., Lachman R.S., Rimoin D.L. Fibrochondrogenesis: Radiologic and histologic studies. Am. J. Med. Genet. 1984;19:277–290. - PubMed
-
- Bankier A., Fortune D., Duke J., Sillence D.O. Fibrochondrogenesis in male twins at 24 weeks gestation. Am. J. Med. Genet. 1991;38:95–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

