Prodynorphin mutations cause the neurodegenerative disorder spinocerebellar ataxia type 23
- PMID: 21035104
- PMCID: PMC2978951
- DOI: 10.1016/j.ajhg.2010.10.001
Prodynorphin mutations cause the neurodegenerative disorder spinocerebellar ataxia type 23
Erratum in
- Am J Hum Genet. 2010 Nov 12;87(5):736
Abstract
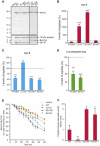
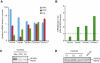
Spinocerebellar ataxias (SCAs) are dominantly inherited neurodegenerative disorders characterized by progressive cerebellar ataxia and dysarthria. We have identified missense mutations in prodynorphin (PDYN) that cause SCA23 in four Dutch families displaying progressive gait and limb ataxia. PDYN is the precursor protein for the opioid neuropeptides, α-neoendorphin, and dynorphins A and B (Dyn A and B). Dynorphins regulate pain processing and modulate the rewarding effects of addictive substances. Three mutations were located in Dyn A, a peptide with both opioid activities and nonopioid neurodegenerative actions. Two of these mutations resulted in excessive generation of Dyn A in a cellular model system. In addition, two of the mutant Dyn A peptides induced toxicity above that of wild-type Dyn A in cultured striatal neurons. The fourth mutation was located in the nonopioid PDYN domain and was associated with altered expression of components of the opioid and glutamate system, as evident from analysis of SCA23 autopsy tissue. Thus, alterations in Dyn A activities and/or impairment of secretory pathways by mutant PDYN may lead to glutamate neurotoxicity, which underlies Purkinje cell degeneration and ataxia. PDYN mutations are identified in a small subset of ataxia families, indicating that SCA23 is an infrequent SCA type (∼0.5%) in the Netherlands and suggesting further genetic SCA heterogeneity.
Copyright © 2010 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Schöls L., Amoiridis G., Büttner T., Przuntek H., Epplen J.T., Riess O. Autosomal dominant cerebellar ataxia: Phenotypic differences in genetically defined subtypes? Ann. Neurol. 1997;42:924–932. - PubMed
-
- Matilla-Dueñas A., Sánchez I., Corral-Juan M., Dávalos A., Alvarez R., Latorre P. Cellular and molecular pathways triggering neurodegeneration in the spinocerebellar ataxias. Cerebellum. 2010;9:148–166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

