Ischemic preconditioning decreases mitochondrial proton leak and reactive oxygen species production in the postischemic heart
- PMID: 21035133
- PMCID: PMC3005326
- DOI: 10.1016/j.jss.2010.09.018
Ischemic preconditioning decreases mitochondrial proton leak and reactive oxygen species production in the postischemic heart
Abstract
Background: Proton leak (H(+) leak) dissipates mitochondrial membrane potential (mΔΨ) through the re-entry of protons into the mitochondrial matrix independent of ATP synthase. Changes in H(+) leak may affect reactive oxygen species (ROS) production. We measured H(+) leak and ROS production during ischemia-reperfusion and ischemic preconditioning (IPC) and examined how changing mitochondrial respiration affected mΔΨ and ROS production.
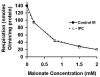
Materials and methods: Isolated rat hearts (n = 6/group) were subjected to either control-IR or IPC. Rate pressure product (RPP) was measured. Mitochondria were isolated at end reperfusion. Respiration was measured by polarography and titrated with increasing concentrations of malonate (0.5-2 mM). mΔΨ was measured using a tetraphenylphosphonium electrode. H(+) leak is the respiratory rate required to maintain membrane potential at -150 mV in the presence of oligomycin-A. Mitochondrial complex III ROS production was measured by fluorometry using Amplex-red.
Results: IPC improved recovery of RPP at end reperfusion (63% ± 4% versus 21% ± 2% in control-IR, P < 0.05). Ischemia-reperfusion caused increased H(+) leak (94 ± 12 versus 31 ± 1 nmol O/mg protein/min in non-ischemic control, P < 0.05). IPC attenuates these increases (55 ± 9 nmol O/mg protein/min, P < 0.05 versus control-IR). IPC reduced mitochondrial ROS production compared with control-IR (31 ± 2 versus 40 ± 3 nmol/mg protein/min, P < 0.05). As mitochondrial respiration decreased, mΔΨ and mitochondrial ROS production also decreased. ROS production remained lower in IPC than in control-IR for all mΔΨ and respiration rates.
Conclusions: Increasing H(+) leak is not associated with decreased ROS production. IPC decreases both the magnitude of H(+) leak and ROS production after ischemia-reperfusion.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures








Similar articles
-
Mitochondrial uncoupling does not decrease reactive oxygen species production after ischemia-reperfusion.Am J Physiol Heart Circ Physiol. 2014 Oct 1;307(7):H996-H1004. doi: 10.1152/ajpheart.00189.2014. Epub 2014 Aug 1. Am J Physiol Heart Circ Physiol. 2014. PMID: 25085966 Free PMC article.
-
Ischemic preconditioning preserves mitochondrial membrane potential and limits reactive oxygen species production.J Surg Res. 2012 Nov;178(1):8-17. doi: 10.1016/j.jss.2012.05.090. Epub 2012 Jun 17. J Surg Res. 2012. PMID: 22763215 Free PMC article.
-
Mitochondrial function during ischemic preconditioning.Surgery. 2002 Feb;131(2):172-8. doi: 10.1067/msy.2002.119490. Surgery. 2002. PMID: 11854695
-
Mitochondrial Proton Leak Plays a Critical Role in Pathogenesis of Cardiovascular Diseases.Adv Exp Med Biol. 2017;982:359-370. doi: 10.1007/978-3-319-55330-6_20. Adv Exp Med Biol. 2017. PMID: 28551798 Free PMC article. Review.
-
Role of reactive oxygen species in ischemic preconditioning of subcellular organelles in the heart.Antioxid Redox Signal. 2004 Apr;6(2):393-404. doi: 10.1089/152308604322899468. Antioxid Redox Signal. 2004. PMID: 15025941 Review.
Cited by
-
Grape seed proanthocyanidins prevent irradiation-induced differentiation of human lung fibroblasts by ameliorating mitochondrial dysfunction.Sci Rep. 2017 Mar 3;7(1):62. doi: 10.1038/s41598-017-00108-9. Sci Rep. 2017. PMID: 28246402 Free PMC article.
-
Pathways for Cardioprotection in Perspective: Focus on Remote Conditioning and Extracellular Vesicles.Biology (Basel). 2023 Feb 14;12(2):308. doi: 10.3390/biology12020308. Biology (Basel). 2023. PMID: 36829584 Free PMC article. Review.
-
Zofenopril Protects Against Myocardial Ischemia-Reperfusion Injury by Increasing Nitric Oxide and Hydrogen Sulfide Bioavailability.J Am Heart Assoc. 2016 Jul 5;5(7):e003531. doi: 10.1161/JAHA.116.003531. J Am Heart Assoc. 2016. PMID: 27381758 Free PMC article.
-
Moving Forwards by Blocking Back-Flow: The Yin and Yang of MI Therapy.Circ Res. 2016 Mar 4;118(5):898-906. doi: 10.1161/CIRCRESAHA.115.306569. Circ Res. 2016. PMID: 26941425 Free PMC article. Review.
-
Nitroalkenes confer acute cardioprotection via adenine nucleotide translocase 1.J Biol Chem. 2012 Jan 27;287(5):3573-80. doi: 10.1074/jbc.M111.298406. Epub 2011 Dec 9. J Biol Chem. 2012. PMID: 22158628 Free PMC article.
References
-
- Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. Am J Physiol Cell Physiol. 2008;294:C460–C466. - PubMed
-
- Lesnefsky EJ, Chen Q, Moghaddas S, Hassan MO, Tandler B, Hoppel CL. Blockade of electron transport during ischemia protects cardiac mitochondria. J Biol Chem. 2004;279:47961–47967. - PubMed
-
- Zweier JL. Measurement of superoxide-derived free radicals in the reperfused heart: evidence for a free radical mechanism of reperfusion injury. J Biol Chem. 1988;263:1353–1357. - PubMed
-
- Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: Central role of complex III. J Biol Chem. 2003;278:36027–36031. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

