Targeting gastrin-releasing peptide as a new approach to treat aggressive refractory neuroblastomas
- PMID: 21035156
- PMCID: PMC3668794
- DOI: 10.1016/j.surg.2010.08.011
Targeting gastrin-releasing peptide as a new approach to treat aggressive refractory neuroblastomas
Abstract
Background: The overall survival for neuroblastoma remains dismal, in part due to the emergence of resistance to chemotherapeutic drugs. We have demonstrated that gastrin-releasing peptide (GRP), a gut peptide secreted by neuroblastoma, acts as an autocrine growth factor. We hypothesized that knockdown of GRP will induce apoptosis in neuroblastoma cells and potentiate the cytotoxic effects of chemotherapeutic agents.
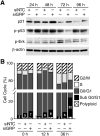
Methods: The human neuroblastoma cell lines (JF, SK-N-SH) were transfected with small interfering (si) RNA targeted at GRP. Apoptosis was assessed by DNA fragmentation assay. Immunoblotting was used to confirm molecular markers of apoptosis, and flow cytometry was performed to determine cell cycle arrest after GRP knockdown.
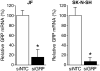
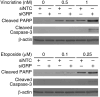
Results: siGRP resulted in an increase in apoptosis in the absence of chemotherapeutic interventions. A combination of GRP silencing and chemotherapeutic drugs resulted in enhanced apoptosis when compared to either of the treatments alone. GRP silencing led to increased expression of proapoptotic proteins, p53 and p21.
Conclusion: Silencing of GRP induces apoptosis in neuroblastoma cells; it acts synergistically with chemotherapeutic effects of etoposide and vincristine. GRP knockdown-mediated apoptosis appears to be associated with upregulation of p53 in neuroblastoma cells. Targeting GRP may be postulated as a potential novel agent for combinational treatment to treat aggressive neuroblastomas.
Copyright © 2011 Mosby, Inc. All rights reserved.
Figures
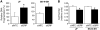


Similar articles
-
Targeting gastrin-releasing peptide suppresses neuroblastoma progression via upregulation of PTEN signaling.PLoS One. 2013 Sep 9;8(9):e72570. doi: 10.1371/journal.pone.0072570. eCollection 2013. PLoS One. 2013. PMID: 24039782 Free PMC article.
-
Gastrin-releasing peptide receptor silencing suppresses the tumorigenesis and metastatic potential of neuroblastoma.Proc Natl Acad Sci U S A. 2008 Sep 2;105(35):12891-6. doi: 10.1073/pnas.0711861105. Epub 2008 Aug 27. Proc Natl Acad Sci U S A. 2008. PMID: 18753628 Free PMC article.
-
Gastrin-releasing peptide is a growth factor for human neuroblastomas.Ann Surg. 2002 May;235(5):621-9; discussion 629-30. doi: 10.1097/00000658-200205000-00003. Ann Surg. 2002. PMID: 11981207 Free PMC article.
-
Gastrin-releasing peptide-induced down-regulation of tumor suppressor protein PTEN (phosphatase and tensin homolog deleted on chromosome ten) in neuroblastomas.Ann Surg. 2005 May;241(5):684-91; discussion 691-2. doi: 10.1097/01.sla.0000161173.47717.71. Ann Surg. 2005. PMID: 15849504 Free PMC article.
-
Role of gastrointestinal hormones in neuroblastoma.World J Surg. 2005 Mar;29(3):281-6. doi: 10.1007/s00268-004-7815-4. World J Surg. 2005. PMID: 15706438 Review.
Cited by
-
Differential regulation of cyclin-dependent kinase inhibitors in neuroblastoma cells.Biochem Biophys Res Commun. 2013 May 31;435(2):295-9. doi: 10.1016/j.bbrc.2013.04.023. Epub 2013 Apr 22. Biochem Biophys Res Commun. 2013. PMID: 23618860 Free PMC article.
-
Significance of gastrin-releasing peptide in ovarian cancer ES2 cells.Oncol Lett. 2015 Jul;10(1):359-363. doi: 10.3892/ol.2015.3240. Epub 2015 May 20. Oncol Lett. 2015. PMID: 26171030 Free PMC article.
-
Bombesin Receptor Family Activation and CNS/Neural Tumors: Review of Evidence Supporting Possible Role for Novel Targeted Therapy.Front Endocrinol (Lausanne). 2021 Sep 1;12:728088. doi: 10.3389/fendo.2021.728088. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34539578 Free PMC article. Review.
-
Autophagy mediates paracrine regulation of vascular endothelial cells.Lab Invest. 2013 Jun;93(6):639-45. doi: 10.1038/labinvest.2013.57. Epub 2013 Apr 22. Lab Invest. 2013. PMID: 23608754 Free PMC article.
-
Targeting gastrin-releasing peptide suppresses neuroblastoma progression via upregulation of PTEN signaling.PLoS One. 2013 Sep 9;8(9):e72570. doi: 10.1371/journal.pone.0072570. eCollection 2013. PLoS One. 2013. PMID: 24039782 Free PMC article.
References
-
- Fan M, Goodwin M, Vu T, Brantley-Finley C, Gaarde WA, Chambers TC. Vinblastine-induced phosphorylation of Bcl-2 and Bcl-XL is mediated by JNK and occurs in parallel with inactivation of the Raf-1/MEK/ERK cascade. J Biol Chem. 2000;275:29980–5. - PubMed
-
- Gomber S, Dewan P, Chhonker D. Vincristine induced neurotoxicity in cancer patients. Indian J Pediatr. 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

