The use of hydrogel microparticles to sequester and concentrate bacterial antigens in a urine test for Lyme disease
- PMID: 21035184
- PMCID: PMC3019571
- DOI: 10.1016/j.biomaterials.2010.10.004
The use of hydrogel microparticles to sequester and concentrate bacterial antigens in a urine test for Lyme disease
Abstract
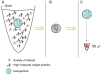
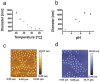
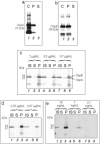
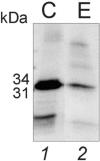
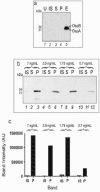
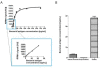
Hydrogel biomarker capturing microparticles were evaluated as a biomaterial to amplify the sensitivity of urine testing for infectious disease proteins. Lyme disease is a bacterial infection transmitted by ticks. Early diagnosis and prompt treatment of Lyme disease reduces complications including arthritis and cardiac involvement. While a urine test is highly desirable for Lyme disease screening, this has been difficult to accomplish because the antigen is present at extremely low concentrations, below the detection limit of clinical immunoassays. N-isopropylacrylamide (NIPAm)-acrylic acid (AAc) microparticles were covalently functionalized with amine containing dyes via amidation of carboxylic groups present in the microparticles. The dyes act as affinity baits towards protein analytes in solution. NIPAm/AAc microparticles functionalized with acid black 48 (AB48) mixed with human urine, achieved close to one hundred percent capture and 100 percent extraction yield of the target antigen. In urine, microparticles sequestered and concentrated Lyme disease antigens 100 fold, compared to the absence of microparticles, achieving an immunoassay detection sensitivity of 700 pg/mL in 10 mL urine. Antigen present in a single infected tick could be readily detected following microparticle sequestration. Hydrogel microparticles functionalized with high affinity baits can dramatically increase the sensitivity of urinary antigen tests for infectious diseases such as Lyme disease. These findings justify controlled clinical studies evaluating the sensitivity and precision of Lyme antigen testing in urine.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures


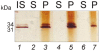
Similar articles
-
Development of a Multiantigen Panel for Improved Detection of Borrelia burgdorferi Infection in Early Lyme Disease.J Clin Microbiol. 2015 Dec;53(12):3834-41. doi: 10.1128/JCM.02111-15. Epub 2015 Oct 7. J Clin Microbiol. 2015. PMID: 26447113 Free PMC article.
-
Linear B Cell Epitopes Derived from the Multifunctional Surface Lipoprotein BBK32 as Targets for the Serodiagnosis of Lyme Disease.mSphere. 2019 May 1;4(3):e00111-19. doi: 10.1128/mSphere.00111-19. mSphere. 2019. PMID: 31043513 Free PMC article.
-
Detection of antigens in urine of mice and humans infected with Borrelia burgdorferi, etiologic agent of Lyme disease.J Clin Microbiol. 1989 Jan;27(1):58-61. doi: 10.1128/jcm.27.1.58-61.1989. J Clin Microbiol. 1989. PMID: 2913036 Free PMC article.
-
Quantitative multiplexed strategies for human Lyme disease serological testing.Exp Biol Med (Maywood). 2021 Jun;246(12):1388-1399. doi: 10.1177/15353702211003496. Epub 2021 Apr 1. Exp Biol Med (Maywood). 2021. PMID: 33794698 Free PMC article. Review.
-
Direct detection methods for Lyme Borrelia, including the use of quantitative assays.Vector Borne Zoonotic Dis. 2002 Winter;2(4):223-31. doi: 10.1089/153036602321653806. Vector Borne Zoonotic Dis. 2002. PMID: 12804163 Review.
Cited by
-
Hydrogel microparticles for biosensing.Eur Polym J. 2015 Nov;72:386-412. doi: 10.1016/j.eurpolymj.2015.02.022. Epub 2015 Feb 28. Eur Polym J. 2015. PMID: 26594056 Free PMC article.
-
Current state of the art for enhancing urine biomarker discovery.Expert Rev Proteomics. 2016 Jun;13(6):609-26. doi: 10.1080/14789450.2016.1190651. Expert Rev Proteomics. 2016. PMID: 27232439 Free PMC article. Review.
-
The use of Nanotrap particles for biodefense and emerging infectious disease diagnostics.Pathog Dis. 2014 Jul;71(2):164-76. doi: 10.1111/2049-632X.12136. Epub 2014 Mar 20. Pathog Dis. 2014. PMID: 24449537 Free PMC article. Review.
-
Photoactive hydrogels for pre-concentration, labelling, and controlled release of proteins.Analyst. 2023 Aug 21;148(17):4127-4137. doi: 10.1039/d3an00811h. Analyst. 2023. PMID: 37493470 Free PMC article.
-
Application of Nanotrap technology for high sensitivity measurement of urinary outer surface protein A carboxyl-terminus domain in early stage Lyme borreliosis.J Transl Med. 2015 Nov 4;13:346. doi: 10.1186/s12967-015-0701-z. J Transl Med. 2015. PMID: 26537892 Free PMC article.
References
-
- Hubalek Z. Epidemiology of Lyme borreliosis. Curr Probl Dermatol. 2009;37:31–50. - PubMed
-
- Cameron DJ. An appraisal of “chronic Lyme disease”. N Engl J Med. 2008;358(4):429–430. - PubMed
-
- Fallon BA, Lipkin RB, Corbera KM, Yu S, Nobler MS, Keilp JG, et al. Regional cerebral blood flow and metabolic rate in persistent Lyme encephalopathy. Arch Gen Psychiatry. 2009;66(5):554–563. - PubMed
-
- Heckler AK, Shmorhun D. Asymptomatic, transient complete heart block in a pediatric patient with Lyme disease. Clin Pediatr (Phila) 2009;49(1):82–85. - PubMed
-
- Barr WB, Rastogi R, Ravdin L, Hilton E. Relations among indexes of memory disturbance and depression in patients with Lyme borreliosis. Appl Neuropsychol. 1999;6(1):12–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

