Epstein-Barr virus replication linked to B cell proliferation in inflamed areas of colonic mucosa of patients with inflammatory bowel disease
- PMID: 21035384
- PMCID: PMC3052968
- DOI: 10.1016/j.jcv.2010.09.011
Epstein-Barr virus replication linked to B cell proliferation in inflamed areas of colonic mucosa of patients with inflammatory bowel disease
Abstract
Background: Inflammatory bowel disease (IBD) is characterized by chronic inflammation of the gastrointestinal tract. Epstein-Barr virus (EBV) infection is associated with increased disease severity in therapeutically immunosuppressed IBD patients. The role of EBV infection in patients with IBD who are unresponsive to medical therapy is unclear. Anti-viral strategies may be a viable treatment option if severity of EBV infection, reflected in peripheral blood, contributes to IBD progression.
Objectives: We investigated the role of EBV in IBD patients unresponsive to medical therapy by examining EBV reactivation and B-cell proliferation in colonic mucosa.
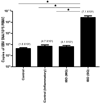
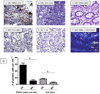
Study design: EBV DNA copy numbers were measured by real-time PCR in peripheral blood mononuclear cells (PBMC) of 84 patients with IBD and 115 non-IBD controls in a retrospective cross-sectional study. EBV-infected cells in colonic mucosa were identified by immunohistochemistry.
Results: EBV load in PBMC was higher in patients with IBD than in non-IBD controls, especially in patients not responding to medication. Inflamed colonic mucosa of these patients had high levels of expression of lytic and latent EBV genes that localized to proliferating B-lymphocytes, which was not seen in patients responding to therapy.
Conclusions: EBV replication was associated with severe IBD and mucosal inflammation. Increased proliferation and EBV infection of B-lymphocytes in inflamed colonic mucosa highlight the potential role of EBV in mucosal inflammation. The immunomodulatory effects of EBV could delay the resolution of the IBD associated inflammation, thus contributing to disease progression. These results indicate that anti-viral therapeutic strategies for the resolution of IBD may be useful.
Copyright © 2010 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors have no conflict of interest in this study.
This material has not been previously presented or published
Figures


References
-
- Macfarlane S, Steed H, Macfarlane GT. Intestinal bacteria and inflammatory bowel disease. Crit Rev Clin Lab Sci. 2009;46(1):25–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

