CD4(+) T cells contribute to the remodeling of the microenvironment required for sustained tumor regression upon oncogene inactivation
- PMID: 21035406
- PMCID: PMC2991103
- DOI: 10.1016/j.ccr.2010.10.002
CD4(+) T cells contribute to the remodeling of the microenvironment required for sustained tumor regression upon oncogene inactivation
Erratum in
- Cancer Cell. 2010 Dec 14;18(6):696
Abstract
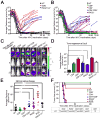
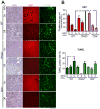
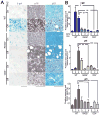
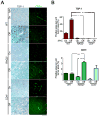
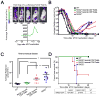
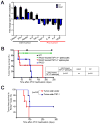
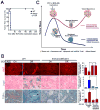
Oncogene addiction is thought to occur cell autonomously. Immune effectors are implicated in the initiation and restraint of tumorigenesis, but their role in oncogene inactivation-mediated tumor regression is unclear. Here, we show that an intact immune system, specifically CD4(+) T cells, is required for the induction of cellular senescence, shutdown of angiogenesis, and chemokine expression resulting in sustained tumor regression upon inactivation of the MYC or BCR-ABL oncogenes in mouse models of T cell acute lymphoblastic lymphoma and pro-B cell leukemia, respectively. Moreover, immune effectors knocked out for thrombospondins failed to induce sustained tumor regression. Hence, CD4(+) T cells are required for the remodeling of the tumor microenvironment through the expression of chemokines, such as thrombospondins, in order to elicit oncogene addiction.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures

Comment in
-
Tumor immunology: CD4(+) T cell sponsor oncogene addicts.Nat Rev Immunol. 2011 Jan;11(1):7. doi: 10.1038/nri2910. Nat Rev Immunol. 2011. PMID: 21218661 No abstract available.
Comment on
-
Can antitumor immunity help to explain "oncogene addiction"?Cancer Cell. 2010 Nov 16;18(5):403-5. doi: 10.1016/j.ccr.2010.11.002. Cancer Cell. 2010. PMID: 21075303 Free PMC article.
References
-
- Acosta JC, O’Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. - PubMed
-
- Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66:1–9. - PubMed
-
- Beatty G, Paterson Y. IFN-gamma-dependent inhibition of tumor angiogenesis by tumor-infiltrating CD4+ T cells requires tumor responsiveness to IFN-gamma. J Immunol. 2001;166:2276–2282. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

