DHHC protein-dependent palmitoylation protects regulator of G-protein signaling 4 from proteasome degradation
- PMID: 21035448
- PMCID: PMC2995692
- DOI: 10.1016/j.febslet.2010.10.052
DHHC protein-dependent palmitoylation protects regulator of G-protein signaling 4 from proteasome degradation
Abstract
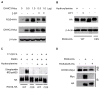
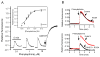
Regulator of G-protein signaling 4 (RGS4), an intracellular modulator of G-protein coupled receptor (GPCR)-mediated signaling, is regulated by multiple processes including palmitoylation and proteasome degradation. We found that co-expression of DHHC acyltransferases (DHHC3 or DHHC7), but not their acyltransferase-inactive mutants, increased expression levels of RGS4 but not its Cys2 to Ser mutant (RGS4C2S). DHHC3 interacts with and palmitoylates RGS4 but not RGS4C2S in vivo. Palmitoylation prolongs the half-life of RGS4 by over 8-fold and palmitoylated RGS4 blocked α(1A)-adrenergic receptor-stimulated intracellular Ca(2+) mobilization. Together, our findings revealed that DHHC proteins could regulate GPCR-mediated signaling by increasing RGS4 stability.
Copyright © 2010 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures



Similar articles
-
Amino-terminal cysteine residues differentially influence RGS4 protein plasma membrane targeting, intracellular trafficking, and function.J Biol Chem. 2012 Aug 17;287(34):28966-74. doi: 10.1074/jbc.M112.345629. Epub 2012 Jun 29. J Biol Chem. 2012. PMID: 22753418 Free PMC article.
-
Palmitoylation of a conserved cysteine in the regulator of G protein signaling (RGS) domain modulates the GTPase-activating activity of RGS4 and RGS10.J Biol Chem. 1999 Dec 31;274(53):38260-7. doi: 10.1074/jbc.274.53.38260. J Biol Chem. 1999. PMID: 10608901
-
Identification of G protein alpha subunit-palmitoylating enzyme.Mol Cell Biol. 2009 Jan;29(2):435-47. doi: 10.1128/MCB.01144-08. Epub 2008 Nov 10. Mol Cell Biol. 2009. PMID: 19001095 Free PMC article.
-
Allosteric regulation of GAP activity by phospholipids in regulators of G-protein signaling.Methods Enzymol. 2004;389:89-105. doi: 10.1016/S0076-6879(04)89006-2. Methods Enzymol. 2004. PMID: 15313561 Review.
-
Structure and function of DHHC protein S-acyltransferases.Biochem Soc Trans. 2017 Aug 15;45(4):923-8. doi: 10.1042/BST20160304. Epub 2017 Jun 19. Biochem Soc Trans. 2017. PMID: 28630137 Review.
Cited by
-
A finer tuning of G-protein signaling through regulated control of RGS proteins.Am J Physiol Heart Circ Physiol. 2012 Jul;303(1):H19-35. doi: 10.1152/ajpheart.00764.2011. Epub 2012 Apr 27. Am J Physiol Heart Circ Physiol. 2012. PMID: 22542620 Free PMC article. Review.
-
Amino-terminal cysteine residues differentially influence RGS4 protein plasma membrane targeting, intracellular trafficking, and function.J Biol Chem. 2012 Aug 17;287(34):28966-74. doi: 10.1074/jbc.M112.345629. Epub 2012 Jun 29. J Biol Chem. 2012. PMID: 22753418 Free PMC article.
-
Understanding Protein Palmitoylation: Biological Significance and Enzymology.Sci China Chem. 2011 Dec;54(12):1888-1897. doi: 10.1007/s11426-011-4428-2. Sci China Chem. 2011. PMID: 25419213 Free PMC article.
-
Protein Lipidation by Palmitate Controls Macrophage Function.Cells. 2022 Feb 6;11(3):565. doi: 10.3390/cells11030565. Cells. 2022. PMID: 35159374 Free PMC article. Review.
-
RGS4 Overexpression in Lung Attenuates Airway Hyperresponsiveness in Mice.Am J Respir Cell Mol Biol. 2018 Jan;58(1):89-98. doi: 10.1165/rcmb.2017-0109OC. Am J Respir Cell Mol Biol. 2018. PMID: 28853915 Free PMC article.
References
-
- Ross EM, Wilkie TM. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu Rev Biochem. 2000;69:795–827. - PubMed
-
- Cifelli C, Rose RA, Zhang H, Voigtlaender-Bolz J, Bolz SS, Backx PH, Heximer SP. RGS4 regulates parasympathetic signaling and heart rate control in the sinoatrial node. Circ Res. 2008;103:527–535. - PubMed
-
- Hooks SB, Martemyanov K, Zachariou V. A role of RGS proteins in drug addiction. Biochem Pharmacol. 2008;75:76–84. - PubMed
-
- Hurst JH, Hooks SB. Regulator of G-protein signaling (RGS) proteins in cancer biology. Biochem Pharmacol. 2009;78:1289–1297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

