Neural heterogeneities influence envelope and temporal coding at the sensory periphery
- PMID: 21035523
- PMCID: PMC4529325
- DOI: 10.1016/j.neuroscience.2010.10.061
Neural heterogeneities influence envelope and temporal coding at the sensory periphery
Abstract
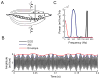
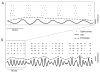
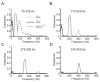
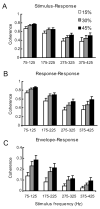
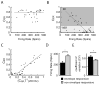
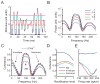
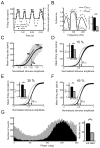
Peripheral sensory neurons respond to stimuli containing a wide range of spatio-temporal frequencies. We investigated electroreceptor neuron coding in the gymnotiform wave-type weakly electric fish Apteronotus leptorhynchus. Previous studies used low to mid temporal frequencies (<256 Hz) and showed that electroreceptor neuron responses to sensory stimuli could be almost exclusively accounted for by linear models, thereby implying a rate code. We instead used temporal frequencies up to 425 Hz, which is in the upper behaviorally relevant range for this species. We show that electroreceptors can: (A) respond up to the highest frequencies tested and (B) display strong nonlinearities in their responses to such stimuli. These nonlinearities were manifested by the fact that the responses to repeated presentations of the same stimulus were coherent at temporal frequencies outside of those contained in the stimulus waveform. Specifically, these consisted of low frequencies corresponding to the time varying contrast or envelope of the stimulus as well as higher harmonics of the frequencies contained in the stimulus. Heterogeneities in the afferent population influenced nonlinear coding as afferents with the lowest baseline firing rates tended to display the strongest nonlinear responses. To understand the link between afferent heterogeneity and nonlinear responsiveness, we used a phenomenological mathematical model of electrosensory afferents. Varying a single parameter in the model was sufficient to account for the variability seen in our experimental data and yielded a prediction: nonlinear responses to the envelope and at higher harmonics are both due to afferents with lower baseline firing rates displaying greater degrees of rectification in their responses. This prediction was verified experimentally as we found that the coherence between the half-wave rectified stimulus and the response resembled the coherence between the responses to repeated presentations of the stimulus in our dataset. This result shows that rectification cannot only give rise to responses to low frequency envelopes but also at frequencies that are higher than those contained in the stimulus. The latter result implies that information is contained in the fine temporal structure of electroreceptor afferent spike trains. Our results show that heterogeneities in peripheral neuronal populations can have dramatic consequences on the nature of the neural code.
Copyright © 2011 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures

References
-
- Bastian J. Electrolocation.1. How the electroreceptors of Apteronotus albifrons code for moving objects and other electrical stimuli. J Comp Physiol. 1981a;144:465–479.
-
- Bastian J. Electrolocation.2. The Effects of moving objects and other electrical stimuli on the activities of 2 categories of posterior lateral line lobe cells in Apteronotus albifrons. J Comp Physiol. 1981b;144:481–494.
-
- Bastian J. Plasticity in an electrosensory system. I. General features of a dynamic sensory filter. J Neurophysiol. 1996a;76:2483–2496. - PubMed
-
- Bastian J. Plasticity in an electrosensory system. II. Postsynaptic events associated with a dynamic sensory filter. J Neurophysiol. 1996b;76:2497–2507. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

