Glucose stimulation of hypothalamic MCH neurons involves K(ATP) channels, is modulated by UCP2, and regulates peripheral glucose homeostasis
- PMID: 21035764
- PMCID: PMC2998191
- DOI: 10.1016/j.cmet.2010.09.013
Glucose stimulation of hypothalamic MCH neurons involves K(ATP) channels, is modulated by UCP2, and regulates peripheral glucose homeostasis
Abstract
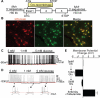
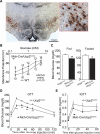
Blood glucose levels are tightly controlled, a process thought to be orchestrated primarily by peripheral mechanisms (insulin secretion by β cells, and insulin action on muscle, fat, and liver). The brain also plays an important, albeit less well-defined role. Subsets of neurons in the brain are excited by glucose; in many cases this involves ATP-mediated closure of K(ATP) channels. To understand the relevance of this, we are manipulating glucose sensing within glucose-excited neurons. In the present study, we demonstrate that glucose excitation of MCH-expressing neurons in the lateral hypothalamus is mediated by K(ATP) channels and is negatively regulated by UCP2 (a mitochondrial protein that reduces ATP production), and that glucose sensing by MCH neurons plays an important role in regulating glucose homeostasis. Combined, the glucose-excited neurons are likely to play key, previously unexpected roles in regulating blood glucose.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity.Nature. 2007 Sep 13;449(7159):228-32. doi: 10.1038/nature06098. Epub 2007 Aug 29. Nature. 2007. PMID: 17728716
-
Increased uncoupling protein (UCP) activity in Drosophila insulin-producing neurons attenuates insulin signaling and extends lifespan.Aging (Albany NY). 2009 Jul 21;1(8):699-713. doi: 10.18632/aging.100067. Aging (Albany NY). 2009. PMID: 20195385 Free PMC article.
-
Diurnal variation of the melanin-concentrating hormone level in the hypothalamus.Acta Biol Hung. 2017 Mar;68(1):14-21. doi: 10.1556/018.68.2017.1.2. Acta Biol Hung. 2017. PMID: 28322083
-
Melanin-concentrating hormone producing neurons: Activities and modulations.Peptides. 2009 Nov;30(11):2031-9. doi: 10.1016/j.peptides.2009.05.028. Epub 2009 Jun 11. Peptides. 2009. PMID: 19524001 Review.
-
The MCH neuron population as a model for the development and evolution of the lateral and dorsal hypothalamus.J Chem Neuroanat. 2016 Sep;75(Pt A):28-31. doi: 10.1016/j.jchemneu.2015.09.004. Epub 2015 Oct 13. J Chem Neuroanat. 2016. PMID: 26459022 Review.
Cited by
-
UCP2, a mitochondrial protein regulated at multiple levels.Cell Mol Life Sci. 2014 Apr;71(7):1171-90. doi: 10.1007/s00018-013-1407-0. Epub 2013 Jun 27. Cell Mol Life Sci. 2014. PMID: 23807210 Free PMC article. Review.
-
Melanin-concentrating hormone neurons promote rapid eye movement sleep independent of glutamate release.Brain Struct Funct. 2019 Jan;224(1):99-110. doi: 10.1007/s00429-018-1766-2. Epub 2018 Oct 3. Brain Struct Funct. 2019. PMID: 30284033 Free PMC article.
-
Molecular Mechanisms of Cancer-Induced Sleep Disruption.Int J Mol Sci. 2019 Jun 6;20(11):2780. doi: 10.3390/ijms20112780. Int J Mol Sci. 2019. PMID: 31174326 Free PMC article. Review.
-
Regulation of Brain Primary Cilia Length by MCH Signaling: Evidence from Pharmacological, Genetic, Optogenetic, and Chemogenic Manipulations.Mol Neurobiol. 2022 Jan;59(1):245-265. doi: 10.1007/s12035-021-02511-w. Epub 2021 Oct 19. Mol Neurobiol. 2022. PMID: 34665407 Free PMC article.
-
Enhancing Retinal Endothelial Glycolysis by Inhibiting UCP2 Promotes Physiologic Retinal Vascular Development in a Model of Retinopathy of Prematurity.Invest Ophthalmol Vis Sci. 2019 Apr 1;60(5):1604-1613. doi: 10.1167/iovs.19-26553. Invest Ophthalmol Vis Sci. 2019. PMID: 30995317 Free PMC article.
References
-
- Ashford ML, Boden PR, Treherne JM. Glucose-induced excitation of hypothalamic neurones is mediated by ATP-sensitive K+ channels. Pflugers Arch. 1990;415:479–483. - PubMed
-
- Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, Vale W, Sawchenko PE. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol. 1992;319:218–245. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

