Impact of ST-246® on ACAM2000™ smallpox vaccine reactogenicity, immunogenicity, and protective efficacy in immunodeficient mice
- PMID: 21036130
- PMCID: PMC3023305
- DOI: 10.1016/j.vaccine.2010.10.039
Impact of ST-246® on ACAM2000™ smallpox vaccine reactogenicity, immunogenicity, and protective efficacy in immunodeficient mice
Abstract
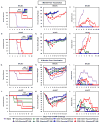
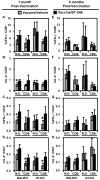
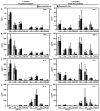
Although a highly effective vaccine against smallpox, vaccinia virus (VV) is not without adverse events, some of which can be life-threatening, particularly in immunocompromised individuals. We have recently demonstrated that the immunogenicity and protective efficacy of Dryvax(®) in immunocompetent mice is preserved even when co-administered with ST-246, an orally bioavailable small-molecule inhibitor of orthopoxvirus egress and dissemination. In addition, ST-246 markedly reduced the reactogenicity of the smallpox vaccine ACAM2000 and the highly neurovirulent VV strain Western Reserve (VV-WR). Here, we evaluated the impact of ST-246 co-administration on ACAM2000 reactogenicity, immunogenicity, and protective efficacy in seven murine models of varying degrees of humoral and cellular immunodeficiency: BALB/c and B-cell deficient (JH-KO) mice depleted of CD4(+) or CD8(+) or both subsets of T cells. We observed that ST-246 reduced vaccine lesion severity and time to complete resolution in all of the immunodeficient models examined, except in those lacking both CD4(+) and CD8(+) T cells. Although VV-specific humoral responses were moderately reduced by ST-246 treatment, cellular responses were generally comparable or slightly enhanced at both 1 and 6 months post-vaccination. Most importantly, in those models in which vaccination given alone conferred protection against lethal VV challenge, similar levels of protection were observed at both time points when vaccination was given with ST-246. These data suggest that, with the exception of individuals with irreversible, combined CD4(+) and CD8(+) T-cell deficiency, ST-246 co-administered at the time of vaccination may help reduce vaccine reactogenicity--even in those lacking humoral immunity--without impeding the induction of protective immunity.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
Immune responses to the smallpox vaccine given in combination with ST-246, a small-molecule inhibitor of poxvirus dissemination.Vaccine. 2008 Feb 13;26(7):933-46. doi: 10.1016/j.vaccine.2007.11.095. Epub 2007 Dec 26. Vaccine. 2008. PMID: 18226434 Free PMC article.
-
Co-administration of tecovirimat and ACAM2000™ in non-human primates: Effect of tecovirimat treatment on ACAM2000 immunogenicity and efficacy versus lethal monkeypox virus challenge.Vaccine. 2020 Jan 16;38(3):644-654. doi: 10.1016/j.vaccine.2019.10.049. Epub 2019 Oct 31. Vaccine. 2020. PMID: 31677948 Free PMC article.
-
Enhancement of CD8+ T cell immunity in the lung by CpG oligodeoxynucleotides increases protective efficacy of a modified vaccinia Ankara vaccine against lethal poxvirus infection even in a CD4-deficient host.J Immunol. 2006 Nov 1;177(9):6336-43. doi: 10.4049/jimmunol.177.9.6336. J Immunol. 2006. PMID: 17056564
-
ACAM2000 clonal Vero cell culture vaccinia virus (New York City Board of Health strain)--a second-generation smallpox vaccine for biological defense.Int J Infect Dis. 2004 Oct;8 Suppl 2:S31-44. doi: 10.1016/j.ijid.2004.09.002. Int J Infect Dis. 2004. PMID: 15491873 Free PMC article. Review.
-
ACAM2000: the new smallpox vaccine for United States Strategic National Stockpile.Drug Des Devel Ther. 2010 May 25;4:71-9. doi: 10.2147/dddt.s3687. Drug Des Devel Ther. 2010. PMID: 20531961 Free PMC article. Review.
Cited by
-
Influence of Population Immunosuppression and Past Vaccination on Smallpox Reemergence.Emerg Infect Dis. 2018 Apr;24(4):646-653. doi: 10.3201/eid2404.171233. Emerg Infect Dis. 2018. PMID: 29553311 Free PMC article.
-
Treatment with the smallpox antiviral tecovirimat (ST-246) alone or in combination with ACAM2000 vaccination is effective as a postsymptomatic therapy for monkeypox virus infection.Antimicrob Agents Chemother. 2015 Jul;59(7):4296-300. doi: 10.1128/AAC.00208-15. Epub 2015 Apr 20. Antimicrob Agents Chemother. 2015. PMID: 25896687 Free PMC article.
-
Co-administration of the broad-spectrum antiviral, brincidofovir (CMX001), with smallpox vaccine does not compromise vaccine protection in mice challenged with ectromelia virus.Antiviral Res. 2014 Nov;111:42-52. doi: 10.1016/j.antiviral.2014.08.003. Epub 2014 Aug 13. Antiviral Res. 2014. PMID: 25128688 Free PMC article.
-
Genomic Analysis, Phenotype, and Virulence of the Historical Brazilian Smallpox Vaccine Strain IOC: Implications for the Origins and Evolutionary Relationships of Vaccinia Virus.J Virol. 2015 Dec;89(23):11909-25. doi: 10.1128/JVI.01833-15. Epub 2015 Sep 16. J Virol. 2015. PMID: 26378174 Free PMC article.
-
ST-246 is a key antiviral to inhibit the viral F13L phospholipase, one of the essential proteins for orthopoxvirus wrapping.J Antimicrob Chemother. 2015 May;70(5):1367-80. doi: 10.1093/jac/dku545. Epub 2015 Jan 27. J Antimicrob Chemother. 2015. PMID: 25630650 Free PMC article.
References
-
- Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. Smallpox and Its Eradication. Geneva, Switzerland: World Health Organization; 1988.
-
- Amanna IJ, Slifka MK, Crotty S. Immunity and immunological memory following smallpox vaccination. Immunol Rev. 2006 Jun;211:320–37. - PubMed
-
- Lane JM, Ruben FL, Neff JM, Millar JD. Complications of smallpox vaccination, 1968: results of ten statewide surveys. J Infect Dis. 1970 Oct;122(4):303–9. - PubMed
-
- Frey SE, Couch RB, Tacket CO, Treanor JJ, Wolff M, Newman FK, et al. Clinical responses to undiluted and diluted smallpox vaccine. N Engl J Med. 2002 Apr 25;346(17):1265–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

