Tumor necrosis factor-α induces sensitization of meningeal nociceptors mediated via local COX and p38 MAP kinase actions
- PMID: 21036476
- PMCID: PMC3391748
- DOI: 10.1016/j.pain.2010.10.002
Tumor necrosis factor-α induces sensitization of meningeal nociceptors mediated via local COX and p38 MAP kinase actions
Abstract
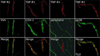
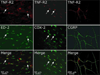
The proinflammatory cytokine TNF-α has been shown to promote activation and sensitization of primary afferent nociceptors. The downstream signaling processes that play a role in promoting this neuronal response remain however controversial. Increased TNF-α plasma levels during migraine attacks suggest that local interaction between this cytokine and intracranial meningeal nociceptors plays a role in promoting the headache. Here, using in vivo single unit recording in the trigeminal ganglia of anesthetized rats, we show that meningeal TNF-α action promotes a delayed mechanical sensitization of meningeal nociceptors. Using immunohistochemistry, we provide evidence for non-neuronal localization of the TNF receptors TNFR1 to dural endothelial vascular cells and TNFR2 to dural resident macrophages as well as to some CGRP-expressing dural nerve fibers. We also demonstrate that meningeal vascular TNFR1 is co-localized with COX-1 while the perivascular TNFR2 is co-expressed with COX-2. We further report here for the first time that TNF-α evoked sensitization of meningeal nociceptors is dependent upon local action of cyclooxygenase (COX). Finally, we show that local application of TNF-α to the meninges evokes activation of the p38 MAP kinase in dural blood vessels that also express TNFR1 and that pharmacological blockade of p38 activation inhibits TNF-α evoked sensitization of meningeal nociceptors. Our study suggests that meningeal action of TNF-α could play an important role in the genesis of intracranial throbbing headaches such as migraine through a mechanism that involves at least part activation of non-neuronal TNFR1 and TNFR2 and downstream activation of meningeal non-neuronal COX and the p38 MAP kinase.
Copyright © 2010 International Association for the Study of Pain. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Vascular extracellular signal-regulated kinase mediates migraine-related sensitization of meningeal nociceptors.Ann Neurol. 2013 Jun;73(6):741-50. doi: 10.1002/ana.23873. Epub 2013 Jun 17. Ann Neurol. 2013. PMID: 23447360 Free PMC article.
-
TNF-α acutely enhances acid-sensing ion channel currents in rat dorsal root ganglion neurons via a p38 MAPK pathway.J Neuroinflammation. 2021 Apr 14;18(1):92. doi: 10.1186/s12974-021-02151-w. J Neuroinflammation. 2021. PMID: 33853615 Free PMC article.
-
Calcitonin gene-related peptide does not excite or sensitize meningeal nociceptors: implications for the pathophysiology of migraine.Ann Neurol. 2005 Nov;58(5):698-705. doi: 10.1002/ana.20619. Ann Neurol. 2005. PMID: 16240341
-
Endogenous mechanisms underlying the activation and sensitization of meningeal nociceptors: the role of immuno-vascular interactions and cortical spreading depression.Curr Pain Headache Rep. 2012 Jun;16(3):270-7. doi: 10.1007/s11916-012-0255-1. Curr Pain Headache Rep. 2012. PMID: 22328144 Review.
-
Response properties of dural nociceptors in relation to headache.J Neurophysiol. 2006 Mar;95(3):1298-306. doi: 10.1152/jn.01293.2005. J Neurophysiol. 2006. PMID: 16492942 Review.
Cited by
-
Sensitization of meningeal afferents to locomotion-related meningeal deformations in a migraine model.Elife. 2024 Feb 8;12:RP91871. doi: 10.7554/eLife.91871. Elife. 2024. PMID: 38329894 Free PMC article.
-
Vascular extracellular signal-regulated kinase mediates migraine-related sensitization of meningeal nociceptors.Ann Neurol. 2013 Jun;73(6):741-50. doi: 10.1002/ana.23873. Epub 2013 Jun 17. Ann Neurol. 2013. PMID: 23447360 Free PMC article.
-
Spreading depression requires microglia and is decreased by their M2a polarization from environmental enrichment.Glia. 2014 Jul;62(7):1176-94. doi: 10.1002/glia.22672. Epub 2014 Apr 10. Glia. 2014. PMID: 24723305 Free PMC article.
-
The glymphatic system in migraine and other headaches.J Headache Pain. 2024 Mar 11;25(1):34. doi: 10.1186/s10194-024-01741-2. J Headache Pain. 2024. PMID: 38462633 Free PMC article. Review.
-
Experimental and Clinical Investigation of Cytokines in Migraine: A Narrative Review.Int J Mol Sci. 2023 May 6;24(9):8343. doi: 10.3390/ijms24098343. Int J Mol Sci. 2023. PMID: 37176049 Free PMC article. Review.
References
-
- Ahn DK, Chae JM, Choi HS, Kyung HM, Kwon OW, Park HS, Youn DH, Bae YC. Central cyclooxygenase inhibitors reduced IL-1beta-induced hyperalgesia in temporomandibular joint of freely moving rats. Pain. 2005;117(1–2):204–213. - PubMed
-
- Constantin CE, Mair N, Sailer CA, Andratsch M, Xu ZZ, Blumer MJ, Scherbakov N, Davis JB, Bluethmann H, Ji RR, Kress M. Endogenous tumor necrosis factor alpha (TNFalpha) requires TNF receptor type 2 to generate heat hyperalgesia in a mouse cancer model. J Neurosci. 2008;28(19):5072–5081. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

